Neural Stem Cells Case File
EUGENE C.TOY, MD, RAHUL JANDIAL, MD, PhD, EVAN YALE SNYDER, MD, PhD, MARTIN T. PAUKERT, MD
CASE 39
A 4-year-old boy is at the amusement park where he complains of increasing headaches and begins to vomit. The parents take him to the children’s hospital where he is evaluated. The family reports that the child had an uncomplicated delivery, received all his immunizations, and is doing well in school. The boy has been experiencing some headaches for the last 2 months, but they were attributed to excessive playing of video games. The radiological investigation reveals a pediatric brain tumor with findings of increased intracranial pressure. The physician tells the boy’s mother that he most likely has a medulloblastoma.
- What part of the brain is most likely affected?
- What is the mechanism for the increased intracranial pressure?
ANSWERS TO CASE 39: NEURAL STEM CELLS
Summary: A 4-year-old boy is evaluated for headaches for 2 months with increasing severity and frequency over the last 24 hours, along with multiple bouts of projectile emesis. The CT scan shows hydrocephalus and a dense mass lesion in the cerebellum. A contrast MRI of the brain is obtained and reveals an enhancing lesion, and most likely a medulloblastoma.
- Part of the brain affected: Posterior fossa.
- Most likely cause of increased intracranial pressure: The obstruction by the tumor of the cerebrospinal outflow via the fourth ventricle led to obstructive hydrocephalus, causing increased intracranial pressure leading to the headaches and emesis. The trigger for emesis can be both from the increased pressure and from tumor compression on an area of the brain stem called the area postrema. The increasing headaches result when the brain’s natural capacity to buffer the increased intracranial pressure is exhausted and small increases in pressure lead to dramatic increase in symptoms.
CLINICAL CORRELATION
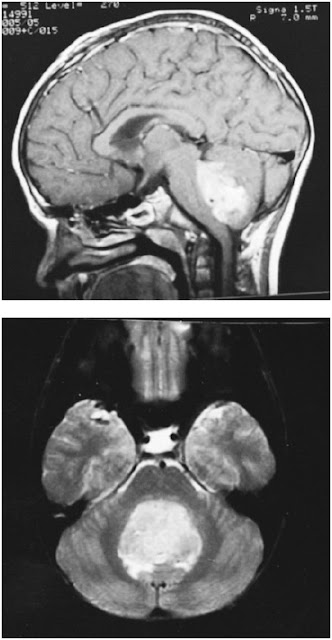
Pediatric brain tumors are one of the most common malignancies in children and have a wide range of histopathology. Some tumors can be removed surgically and with a complete resection, the chance of recurrence is minimal. Also, some tumors are successfully managed with chemotherapy and/or radiation. Yet, a significant portion of pediatric brain tumors is of a highly undifferentiated aggressive histopathology and at the time of diagnosis, often has cells that are outside of the primary tumor and infiltrated the brain. These tumors continue to recur and are managed with repeat operation until the patient ultimately is no longer treatable. Examples of these tumors include medulloblastoma (see Figure 39-1), anaplastic astrocytoma, and anaplastic ependymoma. Furthermore, children under the age of 5 are not candidates for radiation, thereby are even more limited to treatment options. Radiation in children below 5 leads to brain dysfunction.
APPROACH TO NEURAL STEM CELLS
Objectives
- Know the definitions of the various types of stem cells.
- Be able to describe the various sources of neural stem cells.
- Be aware of the limitations and challenges that remain for neural stem cell–based therapy.
Figure 39-1. Medulloblastoma. MRI in the sagittal (above) and axial (below) planes, illustrating involvement of the cerebellar vermis and neoplastic obliteration of the fourth ventricle. (With permission from Adam and Victor’s Principles of Neurology. 7th ed. Figure 31-11, page 703.)
Definitions
Totipotent: Stem cells that can differentiate into embryonic and extraembryonic cell types. After the fusion of an egg and sperm cell, totipotent cells are produced by the first few divisions of the fertilized egg.Pluripotent: Stem cells which can differentiate into any cell within the germ layers. A pluripotent cell can differentiate into any cell of the mesoderm, endoderm, or ectoderm.Multipotent: Stem cells which can differentiate into any cell within its germ line lineage; for example, a neural stem cell exhibits self-renewal and can differentiate into astrocytes, oligodendrocytes, or neurons. It can not, however, differentiate into a cardiomyocyte or intestinal cell.Unipotent: A progenitor cell, which can differentiate into only once cell type such as astrocyte or neuron, but not both. These cells can self-renew and thus are considered stem cells.Embryonic stem cell (ESC): ESCs are cultures of cells derived from the inner cell mass of a blastocyst. A blastocyst is an early stage embryo approximately 4-5 days old in humans and consisting of 50-150 cells. ESCs are pluripotent.Adult (somatic) stem cell: A stem cell that is derived from the fetus, child, or adult and can differentiate into the cells that populate on germ line, and thereby are considered to be multipotent.Epigenetics: Nongenetic influences that influence cells, typically this is the environment in which the cell is found or solution composition in vitro.Transfection: The process of inserting a foreign gene into the host genome of a desired cell. Transfection leads to genetic modification that is stable or transient. Methods include biochemical, physical, and use of viruses.
DISCUSSION
Stem cells give rise to organs and maintain tissue integrity and homeostasis in the adult organism. There are different types of stem cells, including embryonic and somatic (fetal or adult derived) from which new cells can be derived. A stem cell must have the following functional properties: (1) should be able to generate the cell types from the organ it was derived from, and (2) possess “self-renewal,” that is, the ability to produce daughter cells with identical properties. The ability to populate a developing or injured region with appropriate cell types upon transplantation is another important stem cell feature that is well-established with hematopoietic stem cells and awaits standardization in other organ systems including the brain. There are two prototypical stem cells, the embryonic stem cell (ESC) and neural stem cells (NSC).
ESCs have been derived from the inner cell mass of blastocysts of different species including human. They can be totipotent (be able to generate all cells types in an organism except the placenta), pluripotent (the ability to yield mature cell types from all different germ layers), or multipotent (be able to give rise to all cells within an organ). Currently, our understanding of human ESCs is increasing and knowledge is being accumulated on improved cell culture conditions, long-term propagation, controlled differentiation, and transplantation into animal models of human disease. The list of various cell types differentiated from human ESCs (eg, neurons, cardiomyocytes, hepatocytes) is continuously increasing. The unlimited access to specific functional human cells is expected to play not only an important role in therapeutic cell replacement but also for disease modeling and drug screening.
In contrast to pluripotent ESCs, somatic stem cells are believed to be multipotent, thereby capable of generating the major cell types limited to the tissue of origin. Typically, the NSC is capable of producing neurons, astrocytes, and oligodendrocytes. Somatic/tissue-specific stem cells are the building blocks of organs during development and survive in specialized microenvironments (“stem cell niche”) contributing to new cells throughout life. NSCs (1) are multipotent (the ability to yield mature cells in all three fundamental neural lineages throughout the nervous system, neurons; astrocytes, and oligodendrocytes), (2) have the ability to populate a developing region and/or repopulate an ablated or degenerated region of the CNS with appropriate cell types, and (3) undergo “self-renewal”, that is, the ability to produce daughter cells with identical properties. NSCs are highly abundant during embryogenesis, with a sharp decline shortly after birth. In the adult nervous system, NSCs are confined to the subgranular zone (SGZ) in the dentate gyrus of the hippocampus and the subventricular zone (SVZ) lining the lateral ventricles. The newly born neurons in hippocampus have been suggested to improve memory and play a role in mood behavior such as stress and depression. In rodents, neuroblasts born in the SVZ migrate along the rostral migratory stream (RMS) to the olfactory bulbs where they differentiate into periglomerular and granule neurons. Isolation of cells from brain regions such as amygdala, substantia nigra, and cortex has included cells with stem cell characteristics in vitro. Morphologically, NSCs share properties with both astrocytes and radial glia. The main characteristic is a long process that extends radially. Although no definitive marker has been suggested for neural stem cells, a substantial amount of work shows that they are positive for nestin, an intermediary filament protein, and glial fibrillary acidic protein (GFAP), used traditionally to identify astrocytes.
NSCs can be generated from ESCs or directly isolated from the developing CNS (typically from the fetal brain) as well as from neurogenic regions of the adult brain (typically from a cadaveric brain specimen). Historically, the first established NSC lines exploited use of tumor viruses to achieve immortalization. However, NSC that have not been genetically modified can also be propagated in vitro for extended periods of time using high concentrations of mitogenic factors such as basis fibroblast growth factor (bFGF) and epidermal growth factor (EGF).
The challenge to treat brain tumors effectively pivots on the immense difficulty of attacking invading cells within the brain, as well as the delivery of chemotherapeutic modalities past the blood–brain barrier to tumors and tumor cells selectively. The unique ability of NSCs to home tumors has been demonstrated, even when transplanted at various sites outside of the tumor itself. This homing ability has been exploited to deliver therapeutics in various tumor models with remarkable efficacy in mice and may have promise for potential human clinical transplantation. More specifically, nude mice were inoculated with glioma cells and subsequently transplanted with human and murine NSCs at various locations (intratumoral, contralateral hemisphere, intraventricular, and tail vein) with clear demonstration of NSCs migrating to the tumor and distributing within the tumor. Interestingly, some NSCs appeared to track single cells invading brain parenchyma outside of the tumor mass. Subsequently, NSCs were transfected with a gene for cytosine deaminase (CD), a prodrug-converting enzyme that converts 5-FC to 5-FU, and transplanted some distance from the tumor. This technique led to approximately 80% reduction in tumor burden.
Along with the promise of using NSCs to treat brain tumors, issues of patient safety must first be met before clinical trials can occur. Although NSCs are minimally immunogenic, it needs to be defined whether recipients of NSC transplants will need to be on immunosuppressive therapy. Also, the cells transplanted must be able to be followed with imaging in the event they are migrating to unexpected areas outside of the brain. Lastly, the use of genetically modified cells is controversial and concern exists over whether immortalized cells may grow uncontrollably and lead to tumor formation.
Treatment options for an intracranial mass begin with decompressing the hydrocephalus at the bedside with a ventricular catheter. Subsequently, an operation is performed to remove the lesion and obtain histopathological diagnosis. Once tissue diagnosis is obtained, a postoperative plan for adjuvant therapy (radiation and/or chemotherapy) can be created. After resection and adjuvant therapy, most tumors with aggressive histology tend to recur. The treatment options for these recurrent brain tumors is limited and often includes more surgery to decrease the tumor burden, but fails to address the tumor cells that have infiltrated the brain and serve as foci for the seeding and spreading of the primary tumors. The horizon for experimental therapy for recurrent untreatable brain tumors may be the use of neural stem cells. Neural stem cells have demonstrated the ability to migrate toward tumors and tumor cells and in animal models some of these cells were shown to deliver chemotherapeutics and decrease tumor volume.
COMPREHENSION QUESTIONS
[39.1] Neural stems cells can be best described by which of the following terms?
A. TotipotentB. PluripotentC. MultipotentD. Unipotent
[39.2] In the adult brain, in which of the following locations can neural stem cells be found?
A. Ventricular zoneB. Subgranular zoneC. Deep white matterD. Midbrain
[39.3] In which of the following locations can the physician find a totipotent stem cell?
A. A blastocystB. Embryonic mesodermC. The subventricular zone in the human brainD. Skeletal Muscle
Answers
[39.1] C. Neural stems cells are multipotent stem cells. This means that they are capable of dividing to form any of the cells that make up the brain and nervous system, but not cells of any other organ system. The cell lines to which neural stem cells can give rise are neurons, astrocytes, and oligodendrocytes. Recall that other cells that inhabit the CNS and PNS (microglia and Schwann cells) are not derived from neuroectoderm, and therefore cannot be generated by neural stem cells.
[39.2] B. In the adult brain, neural stem cells are located in two different places: the subgranular zone in the hippocampus, and the subventricular zone lining the lateral ventricles. The subgranular zone generates neurons that enter into the dentate gyrus of the hippocampus, and are thought to play a role in improving memory and affecting mood. The subventricular zone generates neurons that migrate to the olfactory bulbs in rodents. The ventricular zone is the location of neural stem cells in the developing nervous system, but they are not located there in the adult nervous system.
[39.3] A. Embryonic stem cells are totipotent early in development, at the blastocyst phase, but begin to restrict their fate soon after that. All the cells in the blastocyst are identical, and any one of them could give rise to any cell in the adult human. By the time a cell has differentiated to embryonic mesoderm, it no longer has the ability to become cells that derive from either endoderm or ectoderm. Neural stem cells in the subventricular zone are multipotent, they can only become cells in the brain, and there are no stem cells in muscle.
|
NEUROSCIENCE
PEARLS
❖ Stem cells are able to indefinitely self-renew as well as
differentiate into various cell types.
❖ Embryonic stem cells can differentiate into any cell and are
thereby pluripotent.
❖ Neural stem cells can differentiate into astrocytes,
oligodendrocytes, or neurons and are thereby multipotent.
❖ Stem cells can be genetically modified to
carry various genes. |
REFERENCES
Falk A, Frisen J. New neurons in old brains. Ann Med. 2005;37:480-486.
Lanza R, Gearhart J, Hogan B. Essentials of Stem Cell Biology. Oxford: Elsevier Academic Press; 2006.
Lindvall OL, Kokaia Z, Martinez-Serrano A. Stem cell therapy for human disorders— how to make it work. Nat Med. 2004;10:S42-S50.

0 comments:
Post a Comment
Note: Only a member of this blog may post a comment.