Neural Repair Case File
EUGENE C.TOY, MD, RAHUL JANDIAL, MD, PhD, EVAN YALE SNYDER, MD, PhD, MARTIN T. PAUKERT, MD
CASE 40
A 24-year-old African American male presents to the emergency room after a car accident resulting in paralysis of both legs. After respiratory stabilization, imaging and neurological assessment take place. Paralysis of both legs is quickly appreciated, and the patient has lost bladder control. Imaging shows a narrowing disruption of the spinal cord at the T11-T12 region of the thoracic spine. A diagnosis of a spinal cord injury (SCI) is made.
- What areas are anatomically affected in spinal cord injury?
- What are the patient’s treatment options?
- Is neural repair a likely outcome for this patient? Why or why not?
ANSWERS TO CASE 40: NEURAL REPAIR
Summary: A 24-year-old African American male presents with paralysis in both legs and a narrowing disruption of the spinal cord at the T11-T12 region of the thoracic spine.
- Regions of spinal cord affected: Spinal cord injury (SCI), also known as myelopathy, results in damage to the white matter tracts (myelinated fiber tracts) in the spinal cord that carry sensation and motor signals to and from the brain. It causes segmental losses of interneurons and motor neurons within the gray matter of the spinal cord.
- Treatment options: First and foremost, every precaution must be taken to stabilize the spine, and not exacerbate the damage following injury. A significant amount of disability in patients with SCI results from insults sustained after the initial injury. Studies have shown that some neurological function can be recovered if methylprednisolone is administered immediately following injury, and this is routinely practiced in the United States. However, at present, there exists no real treatment capable of producing neural repair in the spinal cord. The majority of treatment is focused on rehabilitation and learning to function with disability.
- Is neural repair likely: While axons in the PNS experience spontaneous regeneration, CNS axons do not, making natural recovery from axonal injury in the CNS, such as SCI, impossible. Axon growth and regeneration is a collaborative process that involves regenerative attempts made by the axon itself, and the entire environment surrounding the axon. Unfortunately, the CNS is naturally hostile to axon regeneration. It is riddled with astrocytes, oligodendrocyte progenitors, and oligodendrocytes that all have the ability to inhibit axon regeneration. Additionally, the axon itself does not retain the same vitality in regeneration as it has during developmental growth, and some mismatch between cell surface adhesion molecules on axons and those in the environment exists as well.
CLINICAL CORRELATION
Axon growth and regeneration is a collaborative process that involves regenerative attempts made by the axon itself, and the entire environment surrounding the axon. When a CNS axon is severed, it retracts along with its myelin much like in the PNS. Similar to the PNS, many neurons with severed axons will die, along with their postsynaptic neurons. However, unlike the PNS, the remaining neurons that do survive will atrophy, losing many of the enzymes associated with neurotransmitter production and cellular function. Many of the axons will also lose many of the synaptic connections that link it to the cell body by microglia. Astrocytic processes quickly occupy these vacant connections. Furthermore, in the CNS there is little recruitment of macrophages to enter the axon and remove degenerated axonal and myelin debris except where blood cells and plasma have entered in the area of the lesion itself. Instead, degenerative debris is removed by the growth-inhibiting microglia, which are also present in much smaller numbers. Thus, debris persists in the CNS much longer, inhibiting regeneration. Following the microglia are oligodendrocyte progenitors, and then the lesion site fills up with reactive astrocytes and meningeal cells where the injury penetrates the meningeal surface. These last two types of cells work in tandem to produce a glial scar. All these cell types through these processes have regeneration-inhibiting properties.
APPROACH TO NEURAL REPAIR
Objectives
- Know the effect of damage to CNS in mammals.
- Be aware of the limitations of PNS repair.
- Know the factors that can limit the regeneration.
Definitions
Adhesion molecules: Molecules that regulate cell adhesion by interacting with the molecules on an opposing cell or surface. Adhesion molecules are sometimes referred to as receptors, and their target molecules ligands.Basal lamina sheath: An undamaged axon-Schwann cell unit is surrounded by a basal lamina sheath composed of collagen, laminin, and fibronectin.Band of Bungner: This basal lamina sheath contains tubes of end-to-end Schwann cells that form the band of Bungner which remains intact in the damaged nerve, and, barring mechanical disruption, spans the entire sequence of the nerve from the lesion to the area of axon termination.Perineurial fibroblasts: A sheath of cells that give rise to connective tissue that disperse around a neural lesion.Growth cone: A dynamic actin-supported extension of the developing axon, the growth cone is composed of fine extensions known as filopodia made of actin that contain receptors important for axon guidance.Interleukin 1: Cytokine secreted by axon invading macrophages during PNS repair that induces the NGF production by the Schwann cells which plays a large role in promoting the survival of a neuron following axonal injury.Swollen club ending: The result of halted axonal growth because of fibroblastic scar tissue, also known as a neuroma.NG2: Proteoglycan that inhibits axonal growth released by fibroblasts that make up scar tissue around a neural lesion.
Polyneuronal innervation withdrawal: A process of normal neural development where an initial stage of multineuronal innervations of muscles occur, and is followed by a withdrawal of such connections until each muscle fiber is innervated by only one axon. However, the persistence of this function after development creates positional breaks during regeneration following axonal injury.
DISCUSSION
In mammals, damage to the CNS usually leads to permanent incapacitation of the affected neurons and, in most cases, paralysis. However, the ability to regenerate axons in the peripheral nervous system, and regain much of the function that is lost after peripheral nerve damage, has been retained in mammals. In general, axons regenerate about 1 mm/day. PNS repair is seldom perfect, issues of axon guidance and other factors can limit regeneration.
When a peripheral nerve is damaged, the axons are disconnected from their cell body and begin to degenerate. Schwann cell processes surrounding the axons as myelin also begin to degenerate, and this axonal and myelin debris is removed by macrophages migrating to the degenerating nerve from the bloodstream. Schwann cells undergo several changes during this period including secretion of nerve growth factors NGF and BDNF stimulated by cytokines released by invading macrophages, changes to the Schwann cell membrane surface where adhesion molecules L1, N-CAM, and N-cadherin are
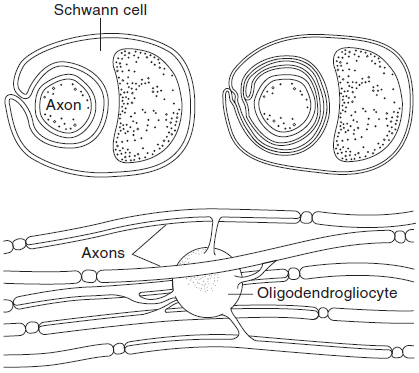
increased, and Schwann cell extracellular matrix transformations occur because of increases in tenascin and several proteoglycans. An undamaged axon-Schwann cell unit is surrounded by a basal lamina sheath composed of collagen, laminin, and fibronectin. This sheath contains tubes of end-to-end Schwann cells that form the band of Bungner which remains intact in the damaged nerve, and, barring mechanical disruption, spans the entire sequence of the nerve from the lesion to the area of axon termination (see Figure 40-1).
The fallout from nerve damage is dependent on the nature of the injury. A major disruption or severance of the nerve leads to complete Schwann cell annihilation leaving just a strip of fibrotic tissue. Conversely, a localized crush injury sufficiently kills axon, but leaves the basal lamina sheath intact. The preservation of the continuity of the Schwann cells seems to be essential for regeneration to occur. However, damage and repair also leads to fibroblastic scarring that can also hinder regeneration. Therefore, neural repair occurs within this context of columns of demyelinated Schwann cells encased in a basal lamina sheath that have changed their surface conformations and have begun to secrete trophic factors, with perineurial fibroblasts dispersed around the lesion. It should be emphasized that nerves regenerate only through intact basal lamina sheaths to ensure that the proper target is reinnervated.
Within an hour of injury, the damaged end of the axon seals off and there is a formation of a growth-cone structure. This is the initial structural response to axonal injury, long before any molecules could have been exchanged between the site of damage and the cell body. For this reason, axons have an inherent ability to form motile growth cones without the production of new molecules. After about a day or two, depending on how far from the cell body the axon was cut, major changes in gene expression and protein synthesis occur in the cell body, and new building block proteins such as tubulin are brought to the axon tip. There is a pattern of expression of cytoskeleton proteins (tubulin) and microtubule-associated proteins in repairing neurons that both mimics and contrasts to neuronal development.
Figure 40-1. Top: Relation of Schwann cells to axons in peripheral nerves. Bottom: Myelination of axons in the central nervous system by oligodendrogliocytes. (With permission from Ganong’s Review of Medical Physiology. 22nd ed, Figure 2-3, page 49.)
Regenerating axons are usually associated with bands of Bungner, and grow in between the basal lamina sheath and the Schwann cell membrane. Half of the growth cone membrane maintains contact with the Schwann cell membrane, and half is connected to the basal lamina. Cell-to-cell interaction during regeneration must necessarily take place between these three elements. In vitro experiments have demonstrated that alone the basal lamina is not sufficient for axonal regeneration; it must be accompanied by Schwann cells. This is because Schwann cells provide the ideal substrate for regenerating axons and supply many of the vital trophic factors that boost nerve repair. Schwann cell division can be promoted by cytokine secretion by macrophages that enter the nerve in response to degeneration to remove axonal and myelin debris. Specifically, interleukin 1 induces NGF production by Schwann cells. Similar changes in Schwann cells, resulting from a disconnect with the axons and spurred by macrophage interactions, make them a suitable substrate for regenerating axons.
Growth cones adhere to nearby surfaces to exert tension on growing axons through adhesion molecules on the growth cone surface, and interactions between substrate adhesion molecules with ligands in the extracellular matrix. L1 and N-cadherin via hemophilic interactions with the axonal surface molecules, and matrix molecules via binding integrins and extracellular matrix all promote axonal growth over Schwann cells. These Schwann cells separated from the axon and stimulated by macrophage secretions will produce many nerve growth factors that play a central role and enhance neural repair. Other extracellular events such as inflammation, which causes the release of many triggering cytokines, also promote axon regeneration.
Some axons grow into the fibroblastic scar tissue that forms at regions of nerve damage. Axonal growth is halted, and a swollen club ending forms. These areas of scar tissue can become extremely sensitive to light touch, and can be quite painful. The more severe the damage, the greater the region of scar tissue formed, and the fewer axons can regenerate. Axons will not regenerate through regions of intense scarring unless the damaged region is surgically removed and replaced by a nerve graft. These regions of scar tissue are so effective at inhibiting axonal growth because of the lack of trophic support, and the large amounts of highly inhibitory proteoglycan NG2 released by fibroblasts.
Neural repair is a directed process because the specificity of axonal connections is required during regeneration to ensure functionality. Regenerating motor axons are expected to find their target muscle, as are sensory axons expected to reconnect to target sensory structures. For this to occur there must be molecular recognition and guidance processes that allow repairing neurons to find their appropriate targets. Much of this specificity is owing to a molecular imprint left behind on the muscle end plate basal lamina. Molecules like agrin and s-laminin, which act as stop signals for axon growth and are found in synaptic junctions, and Schwann cells that envelope the end plate allow repairing neurons to find these old denervated synaptic sites. However, axons still need to be guided by pathways formed by the damaged nerve and its bands of Bungner to the vicinity of the muscle before these effects will take place. An important caveat of muscle reinnervation is that because of lower numbers of regenerating axons, after polyneuronal innervation withdrawal takes place, each nerve fiber ends up innervating more muscle fibers than before, leading to much larger motor units. While this mechanism of reinnervation allows repairing neurons to find old sites of innervations, ensuring that particular axons continue to innervate the same exact sites as before is a much more imperfect science, and many positional errors are generally made by regenerating neurons because of disrupted bands of Bungner. Sensory reinnervation is less understood, regenerated nerve endings are rather different from those that existed before.
Neural repair in the CNS is a new frontier in experimental science since this process does not usually appear in nature. This is because of the nonpermissiveness of the CNS because of astrocyte, oligodendrocyte, and progenitor ability to block axonal growth, and factors intrinsic to the axon itself: the reduced nature of regenerative growth compared to developmental growth, and mismatch between cell surface adhesion molecules on axons and those present in the environment. Strategies to overcome these obstacles in neural repair include replacement of the hostile CNS environment with various grafts, removal of inhibitory cells and molecules, and treatments designed to increase the regenerative capacity of neurons.
COMPREHENSION QUESTIONS
Refer to the following case scenario to answer questions 40.1-40.3:
A 42-year-old man presents to your office complaining of numbness on the dorsal aspect of his right hand and forearm, and difficulty extending his wrist and fingers on that side. On further questioning, he admits that he got drunk and fell asleep with his arm draped at the back of a wooden chair several nights ago. Based on your suspicions, you perform additional tests and confirm that he has a radial neuropathy.
[40.1] Given the nature of this man’s nerve injury, what structure that should span from the distal end of the viable axon to the end of the nerve will allow for nerve regeneration?
A. Band of BungerB. Axon growth coneC. Swollen club endingD. Basal lamina sheath
[40.2] At what rate will the peripheral nerve regenerate, given the appropriate structure to regenerate through?
A. 0.5 mm/dayB. 1 mm/dayC. 2 mm/dayD. 5 mm/day
[40.3] Following peripheral motor nerve regeneration, which of the following best describes the reinnervation pattern of muscles compared to before nerve injury?
A. Each motor axon innervates more muscles.B. Each motor axon innervates fewer muscles.C. Each motor axon innervates the same number of muscles.D. Motor axons do not undergo regeneration.
Answers
[40.1] A. The band of Bunger is a structure made up of Schwann cells and the basal lamina sheath of the endoneurium that used to surround the nerve, and remains intact following injury to the axons of a peripheral nerve. This structure serves as a hollow column down which the damaged axon can grow, and if it is not otherwise damaged, spans from the site of injury to the original endpoint of the nerve, allowing for at least partial regeneration.
[40.2] B. Under optimal conditions, a damaged nerve will regenerate down the hollow center of a band of Bunger at a rate of approximately 1 mm/day. Another commonly used number is 1 in/month.
[40.3] A. When a peripheral nerve regenerates, it does so incompletely, resulting in a smaller number of axons reaching the target than were there originally. This translates into each motor axon innervating a larger number of muscle fibers. Since each motor unit is larger because of the decreased number of axons, the regenerated system has a lesser degree of fine movement control than did the original system.
|
NEUROSCIENCE
PEARLS
❖ Nerves regenerate only through intact basal lamina sheaths to
ensure that the proper target is reinnervated.
❖ Regenerating axons are usually associated with bands of Bungner, and grow in between the basal lamina sheath
and the Schwann cell membrane.
❖ After about a day or two, depending on how far from the cell
body the axon was cut, major changes in gene
expression/protein synthesis occur in the cell body and new building
block proteins such as tubulin are brought to the axon tip.
❖ Because of lower numbers of regenerating axons, after injury,
each nerve fiber ends up innervating more muscle
fibers than before, leading to much larger motor
units. |
REFERENCES
Bear MF, Connors B, Paradiso M, eds. Neuroscience: Exploring the Brain. 3rd ed. Baltimore, MD: Lippincott Williams & Wilkins; 2006.
Purves D, Augustine GJ, Fitzpatrick D, et al, eds. Neuroscience. 3rd ed. Sunderland, MA: Sinauer Associates, Inc.; 2004.
Zigmond MJ, Squire LR, Bloom FE, Landis SC, Roberts JL, eds. Fundamental Neuroscience. 2nd ed. San Diego, CA: Academic Press; 1999.

0 comments:
Post a Comment
Note: Only a member of this blog may post a comment.