Neurogenic Diabetes Insipidus Case File
EUGENE C.TOY, MD, RAHUL JANDIAL, MD, PhD, EVAN YALE SNYDER, MD, PhD, MARTIN T. PAUKERT, MD
CASE 28
A 25-year-old African American female presented to the neurosurgery clinic with an 8 month history of amenorrhea and bitemporal hemianopia. Following laboratory and imaging studies, she was diagnosed with a prolactinoma. After failing bromocriptine therapy for her disease, she elected to undergo transsphenoidal surgical resection of her tumor. Her operation went well and she was doing fine postoperatively until she developed insatiable thirst and began voiding large amounts of urine. Based on her history and symptoms, the diagnosis of neurogenic diabetes insipidus (DI) was made.
- What are the laboratory findings associated with neurogenic DI?
- What are the other causes of neurogenic DI?
ANSWERS TO CASE 28: NEUROGENIC DIABETES INSIPIDUS
Summary: 25-year-old female with a pituitary prolactinoma, who failed medical management and is now stable post-transsphenoidal resection of her tumor with polydipsia and polyuria.
- Laboratory abnormalities: Urine specific gravity less than 1.005 and urinary output greater than 250 mL/hr.
- Other causes: Head trauma, meningitis, encephalitis, autoimmune, familial, idiopathic, neoplastic, and following intracranial procedures.
CLINICAL CORRELATION
The hypothalamus is responsible for various dimensions of homeostasis, one of which is water balance. Vasopressin, or antidiuretic hormone (ADH), is produced in the paraventricular and supraoptic nuclei of the hypothalamus. The neuron cell bodies in these nuclei have axons that descend into the median eminence, pituitary stalk, and then into the posterior lobe of the pituitary gland where they are stored. Plasma osmolality stimulates ADH release directly from the supraoptic and paraventricular nuclei and indirectly from osmoreceptors in other hypothalamic nuclei. Volume-sensitive pathways can also regulate ADH release. Baroreceptors and mechanoreceptors in the aortic arch, carotid sinus, and right atrium can signal for ADH release in times of volume depletion.
ADH stimulates aquaporin channels to open in the luminal membranes of the cortical and medullary collecting tubules of the kidney to promote water reabsorption. Treatment of DI consists of administering synthetic ADH, or DDAVP, to compensate for low levels of ADH.
APPROACH TO REGULATORY FUNCTIONS OF THE HYPOTHALAMUS
Objectives
- Know the names and principle functions of the substances formed by the hypothalamus.
- Know the principle functions of each hypothalamic nucleus.
- Differentiate between DI, SIADH, and cerebral salt wasting.
Definitions
Thyrotropin-releasing hormone (TRH): It stimulates release of thyroidstimulating hormone (TSH) from the anterior pituitary.Growth hormone–releasing hormone (GHRH): It stimulates growth hormone release from anterior pituitary.Corticotropin-releasing hormone (CRH): It stimulates adrenocorticotropic hormone release from anterior pituitary.Gonadotropin-releasing hormone (GnRH): It stimulates luteinizing and follicle-stimulating hormone release from anterior pituitary.Prolactin-releasing hormone: It stimulates prolactin release from the anterior pituitary.Oxytocin: It is produced by the paraventricular and supraoptic nuclei and migrates into the posterior pituitary.
DISCUSSION
The hypothalamus is a bilateral structure that resides on the sides and floor of the third ventricle. It has three general functions: autonomic regulation, endocrine regulation, and circadian regulation. The hypothalamus is comprised of three different zones. The periventricular zone, which borders the third ventricle, has neuroendocrine functions. The medial zone, which is bound laterally by the fornix, functions in autonomic and neuroendocrine control of the enteric system. The lateral zone, which is bound medially by the fornix and laterally by the internal capsule, functions in autonomic and neuroendocrine control of the cardiovascular system. The circuitry of the hypothalamus is very complex, and many of its neuronal connections are bidirectional. It sends and receives information via the blood stream—through the pituitary by way of the hypophyseal-portal system and through circumventricular organs, sites at which the blood–brain barrier is highly permeable and allows passage of chemical stimuli into the brain. The hypothalamus is the key brain site for the integration of multiple biologic systems to maintain homeostasis.
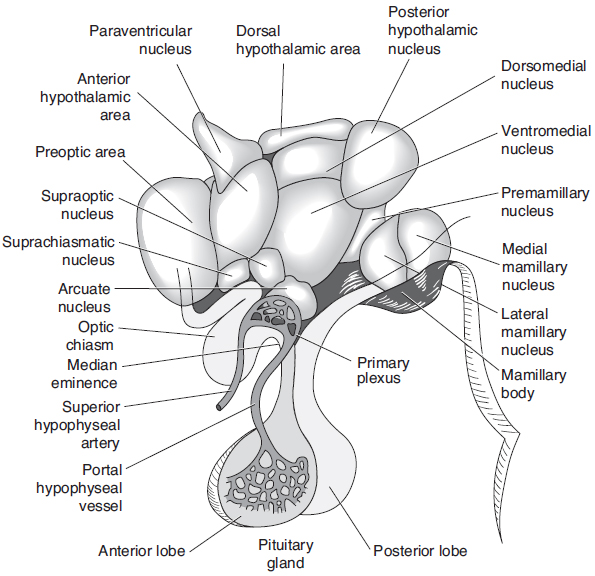
These functions are executed by the various nuclei of the hypothalamus (see Figure 28-1). The paraventricular and supraoptic nuclei have already been discussed. The anterior nucleus is responsible for regulating the dissipation of heat from the body. It also stimulates the parasympathetic nervous system. A lesion will cause hyperthermia. The posterior nucleus regulates heat conservation. It also stimulates the sympathetic nervous system. A lesion will cause hypothermia. A lesion of the dorsomedial nucleus will result in savage behavior. The ventromedial nucleus regulates satiety. A lesion will result in obesity and savage behavior. The preoptic nucleus is responsible for regulating gonadotropin release. The suprachiasmatic nucleus controls circadian rhythms. A lesion will result in disruption of sleep–wake cycles. The lateral nucleus regulates the desire to eat. A lesion will result in starvation. The mamillary body receives input from the hippocampal formation by way of fibers in the fornix. Hemorrhagic lesions are found in the mamillary nuclei in Wernicke’s encephalopathy. The arcuate nucleus has axons projected back to the hypophyseal portal system, where they release dopamine to inhibit prolactin release from the anterior pituitary gland.
Figure 28-1. Human hypothalamus, with a superimposed diagrammatic representation of the portal hypophyseal vessels. (With permission from Ganong’s Review of Medical Physiology. 22nd ed, Figure 14-2, page 223.)
Many authors divide hypothalamic dysfunction into global and partial. In global dysfunction, many or all of the hypothalamic functions are deranged. Common causes are usually systemic in nature. Sarcoidosis, metastatic cancer, idiopathic inflammatory diseases, and germ cell cancers all participate in global hypothalamic dysfunction. Partial hypothalamic dysfunction results when only one of the hormones is disturbed, as in the case of DI.
Syndrome of inappropriate secretion of antidiuretic hormone (SIADH) occurs when there is an unnecessary release of ADH. Common triggers include neurosurgical procedures, head trauma, neoplasms, CNS infections, and autoimmune disorders like Guillain-Barre syndrome. Often the initial finding is dilutional hyponatremia without edema in the setting of low osmolality and high urine specific gravity (> 300 mosm/L). Treatment is simple: free water restriction or in the case of seizures secondary to hyponatremia, 3%
NaCl with sodium correction no greater than 12 mEq/L in the first 24 hours and no greater than 20 mEq/L in 48 hours. This avoids the debilitating sequelae of central pontine demyelinolysis. Another common cause of hyponatremia in neurosurgical patients is cerebral salt wasting. Unlike SIADH, where there is too much water in the serum-diluting sodium, in cerebral salt wasting there is a volume contraction because of loss of salt in the urine. Thus, patients with cerebral salt wasting will have a suboptimal fluid balance and high levels of urine sodium, which is the opposite of SIADH patients. Thus, it is crucial to assess a patient’s volume status and urine sodium before instituting treatment. For example, fluid restricting a patient with presumed SIADH could have detrimental effects on the renal, cardiovascular, and neurological systems of a patient who is actually volume depleted in the setting of cerebral salt wasting.
DI is a disease characterized by excretion of large amounts of severely diluted urine, which cannot be reduced when fluid intake is reduced. It denotes inability of the kidney to concentrate urine. DI is caused by a deficiency of ADH, also known as vasopressin, or by an insensitivity of the kidneys to that hormone. The regulation of urine production occurs in the hypothalamus, which produces ADH (or vasopressin) in the supraoptic and paraventricular nuclei. After synthesis, the hormone is transported in neurosecretory granules down the axon of the hypothalamic neuron to the posterior lobe of the pituitary gland, where it is stored for later release. In addition, the hypothalamus regulates the sensation of thirst in the ventromedial nucleus by sensing increases in serum osmolarity and relaying this information to the cortex. The main effector organ for fluid homeostasis is the kidney. ADH acts by increasing water permeability in the collecting ducts, specifically it acts on proteins called aquaporins which open to allow water into the collecting duct cells. This increase in permeability allows for reabsorption of water into the bloodstream, thus concentrating the urine.
COMPREHENSION QUESTIONS
[28.1] A 32-year-old man presents to your office with a chief complaint of sleep disturbance. He works in a rotating shift at a chemical plant, and changes from days to evenings to nights every week. He states that his problem is that he often cannot fall asleep when he needs to, and is frequently very tired at work. You diagnose him with circadian rhythm sleep disorder and discuss treatment options with him. Which hypothalamic nucleus is normally responsible for controlling the circadian rhythm?
A. Supraoptic nucleusB. Posterior nucleusC. Suprachiasmatic nucleusD. Lateral nucleus
[28.2] A morbidly obese patient comes into your office for an annual checkup. As a responsible physician you inform this patient of the risks associated with excess body fat and discuss some methods for losing weight. The patient states that she has tried “more diets than I can count” and that none of them worked. She states that there must be some underlying problem with her body that prevents her from losing weight. If she had a hypothalamic lesion responsible for her excess weight, where would it be?
A. Paraventricular nucleusB. Lateral nucleusC. Dorsomedial nucleusD. Ventromedial nucleus
[28.3] A 60-year-old woman comes into your office complaining of fatigue, weight gain, and feeling cold all the time. On further questioning she endorses constipation and states that she hasn’t been eating as much as usual, despite the gain in weight. You check her thyroid status, and find low thyroxine and low TSH, indicating a defect either in the pituitary or in the hypothalamus. If the problem is in the hypothalamus, which nucleus would it be affecting?
A. Arcuate nucleusB. Paraventricular nucleusC. Anterior nucleusD. Mamillary bodies
Answers
[28.1] C. The suprachiasmatic nucleus, located just superior to the optic chiasm, is responsible for controlling circadian rhythm. The free running time of the cycle generated by the suprachiasmatic nucleus is about 25 hours, but it receives input directly from the retina, and therefore alters its generated frequency to match the actual length of the day.
[28.2] D. The ventromedial nucleus is considered the “satiety center” of the hypothalamus, and at least in laboratory animals, lesioning this nucleus bilaterally results in animals that eat uncontrollably and become morbidly obese. Because the hypothalamus is so small in humans, however, lesions of a single nucleus, let along bilateral lesions of a nucleus are extremely rare. The lateral nucleus is considered the “feeding center” of the hypothalamus, and bilateral lesions of it in laboratory animals results in animals that have no interest in eating and will actually starve to death with food accessible.
[28.3] A. The arcuate nucleus of the hypothalamus is responsible for secreting factors that stimulate or inhibit release of pituitary hormones. Among these factors is TRH (thyrotropin-releasing hormone), which travels through the hypophyseal portal system to the anterior pituitary where it stimulates thyrotropes to release TSH (thyroid-stimulating hormone). Low TSH in the face of low levels of thyroid hormone either indicates a defect in the thyrotropes of the pituitary, in the arcuate nucleus of the hypothalamus, or in the connection between the two. The paraventricular nucleus sends axons down the pituitary stalk to the posterior lobe of the pituitary where it releases ADH and oxytocin directly into the bloodstream.
|
NEUROSCIENCE
PEARLS
❖ The hypothalamus is crucial to autonomic, endocrine, and
circadian regulation.
❖ ADH is produced in the paraventricular and supraoptic nuclei of
the hypothalamus.
❖ Baroreceptors and mechanoreceptors in the aortic arch, carotid sinus and right atrium signal for ADH
release in times of volume depletion.
❖ DI is a disease characterized by excretion of large amounts of severely diluted urine, which cannot be
reduced when fluid intake is reduced.
❖ Common causes of syndrome of inappropriate SIADH include
neurosurgical procedures, head trauma, neoplasms, CNS
infections, and autoimmune disorders like
Guillain-Barre syndrome. |
REFERENCES
Bear MF, Connors BW, Paradiso MA, eds. Neuroscience: Exploring the Brain. 3rd ed. Baltimore, MD: Lippincott Williams & Wilkins; 2006.
Purves D, Augustine GJ, Fitzpatrick D, et al, eds.Neuroscience. 3rd ed. Sunderland, MA: Sinauer Associates, Inc; 2004.
Zigmond MJ, Squire LR, Bloom FE, Landis SC, Roberts JL, eds. Fundamental Neuroscience. 2nd ed. San Diego, CA: Academic Press; 1999.

0 comments:
Post a Comment
Note: Only a member of this blog may post a comment.