Neurogenesis Case File
EUGENE C.TOY, MD, RAHUL JANDIAL, MD, PhD, EVAN YALE SNYDER, MD, PhD, MARTIN T. PAUKERT, MD
CASE 14
The police officers bring in a 72-year-old unidentified woman whom they found walking aimlessly around the grocery store parking lot. On questioning, she remembers her name and that she is 72, but is not sure where she lives or how to contact her family. Cursory examination reveals that she is wearing clean clothes that are slightly wrinkled and with misaligned buttons. She denies any pain or injury. She cannot recall ever seeing a doctor, commenting that she is “okay” and has always been healthy. On neurological examination, she is unable to recall the month/date/year, mistakenly reciting her date of birth several times, and also has difficulty with arithmetic, word recall, and object-naming tasks. The remainder of the physical examination and laboratory results are normal for her age. Her daughter is found and states that her mother has had difficulty with her memory beginning over 1 year ago, which has slowly progressed over time. She stopped shopping 6 months ago when she could not remember what to buy or to keep her checkbook balanced and organized. Over the last several months, she stopped cooking for herself. Her daughter has had to assist increasingly with her mother’s daily activities. Upon careful consideration of the case, you conclude that the patient suffers from dementia, a degenerative brain disorder that seriously affects a person’s ability to carry out daily activities.
- What is the most common cause of dementia?
- What two abnormal intracellular structures are characteristic of the most common cause of dementia?
- What are risk factors?
ANSWERS TO CASE 14: NEUROGENESIS
Summary: The most common form of dementia among older people is Alzheimer disease (AD), which initially involves the parts of the brain that control thought, memory, and language.
- Most common cause of dementia: AD is the most common of the neurodegenerative disorders, which also includes Parkinson disease (PD), Huntington disease (HD), and amyotrophic lateral sclerosis (ALS). The hallmark of these neurodegenerative disorders is the loss of neuronal function, and eventual neuron death. Often, clinical signs predate any significant neuron loss. In AD, the neuron loss occurs in the hippocampus and cerebral cortices.
- Abnormal structures associated with AD: Extensive research has focused on the two well-known abnormalities found in AD, the amyloid plaques and neurofibrillary tangles. The amyloid plaques contain small, toxic cleavage products from a larger precursor protein, amyloid precursor protein (APP), while the neurofibrillary tangles consist of abnormally phosphorylated versions of the microtubule-associating protein, tau. There has been controversy surrounding whether these protein accumulations were the causative factors or just the molecular debris left behind after a prior molecular insult.
- Risk factors: There are multiple risk factors for AD that can not be altered: age (incidence of up to 50% in those 85 years and older), family history of AD, sex (females are at higher risk), and certain genetic traits (a form of apolipoprotein E4 [Apo E4] increases the risk). The evidence for lifestyle modifiable factors is unfortunately less reliable and somewhat controversial. Head injury, most notably in boxers and other full-contact athletes, has also been postulated to be a risk factor for dementia.
CLINICAL CORRELATIONS
Although scientists are learning more every day, right now they still do not know what causes AD, and there is no cure. A Mini Mental State Examination (MMSE) is given to the patient. It is used to screen for the presence of cognitive impairment over a number of domains. Cognition is defined as mental activity such as memory, thinking, attention, reasoning, decision making, and dealing with concepts. She scored 18 out of 30 possible points, consistent with a diagnosis of moderate AD.
Neurodegeneration is a general term that covers many neurodegenerative diseases, including AD, PD, HD, and ALS. Neurodegeneration can also result from stroke, heart attack, head and spinal cord trauma, and bleeding in the brain. Neurodegenerative diseases are often characterized by a neurological deficit, a loss of memory, mobility, independence, or well-being. Each disease has its own characteristics, but all progress slowly over time, and all invariably result in premature death. AD is the most common neurodegenerative disorder worldwide, with approximately 4.5 million affected individuals in the United States alone. It is also the most common cause of cognitive decline, or dementia, in the elderly. From time of diagnosis, life expectancy averages 8 years. Diagnosis hinges upon excluding other conditions that can cause cognitive impairment: small undetected strokes, depression, medications’ side effects, and even PD (another neurodegenerative disorder) has an associated dementia complex. Because AD can only be 100% accurately diagnosed by autopsy, the clinical diagnosis of AD relies upon a clinical picture composed of medical history, blood tests (to rule out other medical conditions), mental status evaluation, neuropsychological testing, and possibly brain imaging (see Figure 14-1). The current interest in treating neurodegenerative diseases like AD with cell-based technologies has renewed the importance of understanding the developmental biology involved with how neurons are formed in the first place. To recapitulate the developmental process, science must first understand that process. Neurogenesis, or the regulated generation of neurons involves (1) early discrimination of neuron from nonneuron, (2) acquisition of specialized neuronal properties, and (3) determination of which cells will live or die as part of development.
APPROACH TO NEUROGENESIS
Objectives
- Understand the cell–cell interactions that progressively restrict the fate of a cell.
- Recognize the role of spatial patterning and temporal regulation in determining cellular identity.
- Be able to describe the molecular mechanisms that regulate the early processes in neurogenesis.
Definitions
Neurogenesis: The process of generating a neuron from a population of neuroepithelial sheet of cells.Progenitor cells: Neuroepithelial or neural stem cells have the capability for long-term self-renewal and can generate all the cell types in the nervous system.Ventricular zone (VZ): The area bordering the ventricular space within the neural tube. This is where the neuroepithelial cells proliferate and differentiate into neurons and migrate away.Subventricular zone (SVZ): A second zone of proliferating cells that continues to proliferate and generate neurons and glia at later stages in development, even into adulthood. It supplies neurons to the olfactory region via the rostral migratory stream.Proneural genes: A family of basic helix-loop-helix (bHLH) transcription factors that is critical to determining the identity of the neural lineage. Neurogenin 1 and 2 are two mammalian examples.Lateral inhibition: The process of cell–cell interaction whereby one cell, destined to a neural lineage, inhibits its neighbor from acquiring the same neural fate.
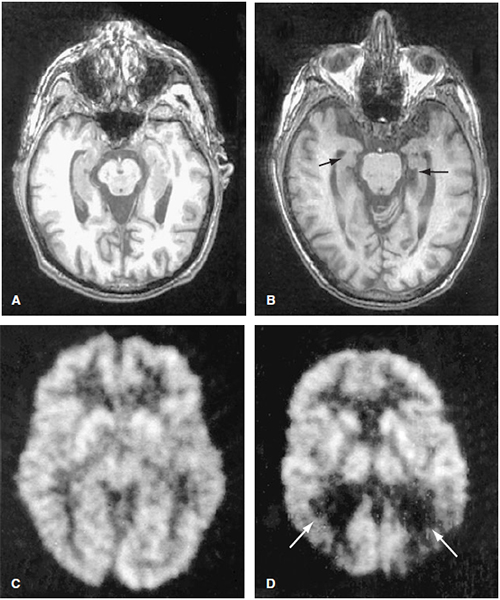
Figure 14-1. Alzheimer disease: Axial T1-weighted MR images through the midbrain of a normal 86-year-old athletic individual (A) and a 77-year-old male (B) with AD. Fluorodexyglucose PET scans of a normal control (C) and a patient with AD (D). Note that the patient with AD has decreased activity in the parietal lobes bilaterally (arrows), a typical finding in this condition. AD, Alzheimer’s disease; PET, positron emission tomography. (With permission from Harrison’s Principles of Internal Medicine. 15th ed. Figure 362-1, page 2392.)
DISCUSSION
The early cellular process of neurogenesis is generally viewed as the progression from multipotent stem cells to fate-restricted neuronal precursors, through the gradual reduction of potential fates. Though the multipotent neuroepithelial cells have long processes that span the width of the early neural tube, all cell division occurs at the ventricular surface. A symmetric cell division is important for self-renewal and critical for early expansion of the population. Later, neural progenitors are produced by asymmetric cell division, which results in the loss of the ability to self-renew, and consequently the cells become more fate restricted. Neural progenitors then begin to express genes that promote differentiation. The last step is to leave the cell cycle (mitosis) and become a neuron.
As the nervous system goes through neural induction, spatial patterning is established along the rostrocaudal and dorsoventral axes. This imprints positional identity upon the neuroepithelial cells, which influences the types of neurons that can arise from the precursors, a fate restriction. For example, isolated cells from the spinal region give rise to cells that populate the spine, whereas cells from the forebrain generate cell types appropriate for the cerebral cortex. A similar process occurs along the dorsoventral axis with these progenitors forming dorsal sensory interneurons and ventral motor neurons. Temporal regulation of neurogenesis affects the neuroprogenitor population as well. In the developing cortex, newly born neurons leave the ventricular zone and migrate along specialized glial cells to populate the cortical layers. Neurons migrate from the ventricular zone to the subventricular zone to their destination via radial glial migration. The glial cells have long processes that extend perpendicular to the subventricular zone and form scaffolding for migrating neurons. Interestingly, the time at which the neurons become postmitotic and leave the cell cycle, their “birthdate,” correlates precisely with their laminar position. Neurons born earlier reside in the deeper layers, while those that are born later populate the more superficial layers. Transplantation studies demonstrated that the early cortical progenitor cells are multipotent and competent to make both early (deep layer) and late (superficial layer) cell types, whereas later progenitors are restricted in their competence and only make the late cell types.
The molecular mechanisms that regulate neurogenesis, like many developmental programs, constitute a balance of forces: forces that promote neural identity and those that constrain the differentiation process. What molecular events are responsible for driving ectodermal cells to become neurons? The first event is to define a subset of cells that can have the potential or competence to become neural precursors. This cluster of cells within the embryological ectoderm, termed a proneural cluster, all express low levels of proneural genes. Through the process of lateral inhibition, neural precursor cells inhibit the expression of proneural genes in neighboring cells, thus preventing them from assuming a neural fate. On a molecular level, this cell–cell interaction is mediated by the Notch-Delta signaling system. The neural precursor cell expresses Delta ligand that activates the Notch receptor on adjacent cells. This activity downregulates the proneural genes and Delta ligand expression in the neighboring cell. This decreased Delta expression in the neighboring cell, by release of inhibition, results in the upregulation of proneural gene expression in the precursor cell, fate-restricting it to becoming a neural precursor cell.
There is no cure for AD. The two drug classes approved by the FDA to help delay the progression of dementia symptoms are cholinesterase inhibitors and memantine. Cholinesterase inhibitors work by blocking the degradation of acetylcholine (Ach), thereby increasing Ach levels in the synaptic cleft. Memantine blocks the NMDA receptor which is thought to be responsible for glutamate-mediated excitotoxicity.
COMPREHENSION QUESTIONS
Refer to the following case scenario to answer questions 14.1-14.3:
A 27-year-old man is evaluated in your office for persistent headaches that are unresponsive to medications and the new onset of instability when walking. An MRI of his head shows an epidermoid cyst (ectopic ectodermal mass) at his cerebellopontine angle. He is referred to neurosurgery for surgical excision.
[14.1] The expression of what gene set in some embryological ectodermal cells causes them to develop into neural tissue rather than ectoderm?
A. Hox genes
B. Proneural genes
C. BMP genes
D. Wnt genes
[14.2] In addition to the developmental signals discussed above, what process aids in the differentiation of some ectoderm into neuroectoderm while adjacent cells continue to differentiate into ectoderm?
A. Lateral inhibition
B. Neurulation
C. Cell fate determination
D. Apoptosis
[14.3] What signaling molecule/receptor pair is responsible for the process of lateral inhibition?
A. Slit/Robo
B. Semaphorin/plexin
C. Notch/Delta
D. Laminin/integrin
Answers
[14.1] B. Proneural genes are necessary for the differentiation of ectodermal cells into neural precursor cells. The differentiation of the embryological ectoderm into neuroectoderm is a very complicated
process that depends on a complex interplay of numerous signaling molecules. Certain embryological ectodermal cells express proneural genes, which are necessary for the differentiation of the cells into neural precursor cells. These proneural genes are inhibited by a family of proteins known as bone morphogenic proteins (BMPs). Signals from embryological mesoderm like chordin, noggin, and follistatin, antagonize the action of BMPs, allowing the expression of the proneural genes and development of embryonic ectoderm into neuroectoderm. Hox genes are involved in a number of embryological developmental stages, including differentiation of the rhombencephalon. Wnt genes are involved in closing of the neural groove into the neural plate.
[14.2] A. Through the process of lateral inhibition, proneural cells secrete signaling molecules that downregulate proneural gene expression in neighboring cells, thereby decreasing the likelihood that those cells will become neuroectodermal cells. While this process does somewhat restrict the fate of the cells that are prevented from becoming neural cells, lateral inhibition is a more specific answer than cell fate determination, and therefore the best choice. Neurulation is the process by which the neural plate becomes the neural tube. Apoptosis (programmed cell death) is a process involved in many other developmental processes.
[14.3] C. The Notch-Delta signaling system is responsible for lateral inhibition. Proneural cells secrete delta ligand, which binds to the notch receptor on neighboring cells, resulting in decreased expression of proneural genes. This also causes a decrease in delta expression in those inhibited cells, thereby relieving inhibition on the initial proneural cell. The other combinations of signaling molecules are involved in axonal guidance.
|
NEUROSCIENCE
PEARLS
❖ During neurogenesis, a multipotent precursor cell goes through a series of developmental steps that
sequentially restrict its potential fates.
❖ The timing of a neuron’s birth is an important predictor of its
cellular fate.
❖ The spatial location of a neuron helps to determine its
identity.
❖ Cell–cell interactions during early neurogenesis are critical
for specifying neural cell fate. |
REFERENCES
Bossy-Wetzel E, Schwarzenbacher R, Lipton SA. Molecular pathways to neurodegeneration. Nat Med. 2004 Jul;10(suppl):S2-S9.
Kandel E, Schwartz J, Jessell T, eds. Principles of Neural Science. 4th ed. New York: McGraw-Hill; 1991.
Lindvall O, Kokaia Z, Martinez-Serrano A. Stem cell therapy for human neurodegenerative disorders—how to make it work. Nat Med. 2004 Jul;10(suppl):S42-S50.
Rao MS, Jacobson M, eds. Developmental Neurobiology. 4th ed. New York: Kluwer Academic/Plenum Publishers; 2005.

0 comments:
Post a Comment
Note: Only a member of this blog may post a comment.