Ataxia, Spinocerebellar Case File
Eugene C. Toy, MD, Ericka Simpson, MD, Pedro Mancias, MD, Erin E. Furr-Stimming, MD
CASE 5
A 57-year-old man of Portuguese descent noticed that he had difficulty marching in line as a soldier. From age 20 until the age of 40 he had a slow progression of symptoms. Since then, he has experienced a rapidly progressive gait disturbance, diplopia, dyssynergia, and paraesthesias in the limbs. At age 45, he was confined to a wheelchair.
On examination, he is intellectually normal but has severe dysarthria and constant drooling. He has bulging eyes, slow saccades, and impaired voluntary up- and downgaze but no nystagmus. He has fasciculations and dyscoordination of the tongue but no facial fasciculations. A general moderate muscle weakness and atrophy are revealed, but muscle tone is normal. Deep tendon reflexes are absent and Babinski signs are present bilaterally. Vibration and proprioception are impaired. Severe ataxia, dysmetria, and dysdiadochokinesia are present. A constant static tremor is seen in the hands.
His mother and maternal grandfather as well as his sister and her son also had problems with gait, which were progressive and began during adulthood. Magnetic resonance imaging (MRI) of the brain reveals cerebellar atrophy.
▶ What is the most likely diagnosis?
▶ What is the next diagnostic step?
▶ What is the next step in therapy?
ANSWERS TO CASE 5:
Ataxia, Spinocerebellar
Summary: This is a case of a previously healthy man who had the insidious onset and gradual progression of a syndrome heralded by gait difficulties, which was later characterized as ataxia.
- Most likely diagnosis: Autosomal dominant cerebellar degeneration with additional neurologic features with normal cognition—most likely spinocerebellar ataxia type 3 (SCA-3).
- Next diagnostic step: DNA testing for dominant ataxias.
- Next step in therapy: Supportive care, genetic counseling, and rehabilitation.
- Describe the movement disorder of ataxia.
- List the differential diagnosis of ataxia, including genetic and nongenetic etiologies.
Considerations
This patient was an otherwise healthy man who had an insidious onset and gradual progression of a syndrome that started with gait difficulties later characterized as ataxia. It progressed to cause dysarthria, abnormal saccades, lower motor neuron findings, a sensory large-fiber neuropathy, and upper motor neuron findings. This clinical picture suggests that multiple systems are involved with the most prominent features being ataxia and poor coordination of voluntary movements. These can be caused by problems with motor control because of pathology of the cerebellum and cerebellar connections, and impaired proprioception occurs because of involvement in these sensory pathways.
Chronic ataxias can present in isolation or in conjunction with other neurologic abnormalities or involvement of other body systems. This patient has other neurologic abnormalities but no evidence of other systemic involvement. In addition, there is a strong suggestion for this being an inherited condition; specifically, there are four successive generations affected in his family, with both sexes affected. This inheritance pattern suggests an autosomal dominant disorder. This is reinforced in that autosomal recessive ataxias tend to have other body systems involved, whereas adult-onset autosomal dominant disease do not.
The differential diagnosis of chronic ataxias includes disorders that affect the cerebellum intrinsically and extrinsically. Intrinsic disorders include infarcts, hemorrhage, toxins such as ethanol and phenytoin, trauma, primary cerebellar neoplasms, metastatic, autoimmune/demyelinating disorders, and remote effects of radiation. Extrinsic factors include paraneoplastic cerebellar degenerations
(PCDs) associated with specific tumor-type antineuronal antibodies. This is a relatively frequent cause of late-onset ataxia and is characterized by a subacute progressive course and requires prompt diagnosis and treatment of the underlying neoplasm. A link between gluten sensitivity and some sporadic ataxias has been suggested due to the high prevalence of presence of antigliadin antibodies, but this remains controversial. Other causes of chronic ataxias are vestibular disorders and sensory neuropathies due to involvement of the peripheral nerves or spinocerebellar tracts as seen with acquired or inherited axonal or demyelinating disorders (ataxia telangiectasia, Friedreich ataxia) and vitamin deficiency (B12). In addition to hormonal and nutritional causes, abnormalities such as hypothyroidism and vitamin E deficiencies can cause ataxia.
APPROACH TO:
Autosomal Dominant Cerebellar Ataxia
DEFINITIONS
ATAXIA: An unsteady and clumsy motion of the limbs or torso caused by a failure of the gross coordination of muscle movements.
TRINUCLEOTIDE REPEAT EXPANSION DISEASE: Caused by stretches of DNA in a gene that contains the same trinucleotide sequence repeated many times. These repeats are a subset of unstable microsatellite repeats that occur throughout all genomic sequences. If the repeat is present in a gene, an expansion of the repeat results in a defective gene product and often leads to disease.
CLINICAL APPROACH
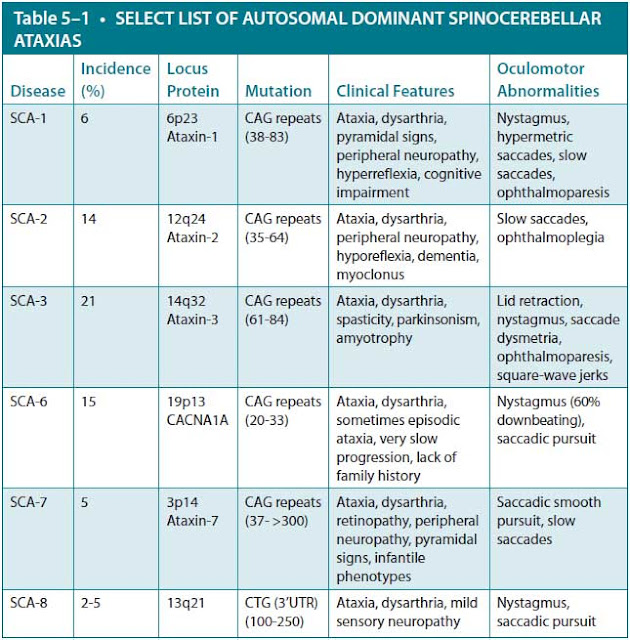
Autosomal dominant cerebellar ataxias (ADCAs) are characterized in terms of their genetic locus and are referred to as spinocerebellar ataxias (SCAs). At this point, there are 43 such disorders, and the number continues to increase. The most common types are listed in Table 5–1. Many of these can be definitively diagnosed by DNA genetic testing. Clinical phenotype and ethnic origin/geographic location can be helpful in prioritizing the order of genetic testing.
There are several gene mutations on different chromosomes that cause SCA, and the gene frequency within different populations varies considerably. In general, the incidence is thought to be approximately 1 to 5 per 100,000 people, with equal gender distribution. Most of the ADCAs are caused by a genetic defect that involves an expansion in the DNA sequence, and most of these are trinucleotide repeat expansions (SCA types 1-3, 6-10, 12, and 17). Other types of repeat expansions that cause SCA have been discovered. For example, SCA-10 involves an ATTCT repeat expansion of the SCA10 gene, and SCA-8 involves an expansion in the SCA8 gene with the nucleotides CTG repeated. Finally, SCA-4 involves a mutation in a gene that does not involve a trinucleotide repeat expansion.
The average age of onset for all of these types is from 20 to 30 years except for SCA-6, which usually occurs between the ages of 40 and 50. People with SCA-8 usually develop symptoms in their late 30s. SCA-2 patients usually develop dementia and slow eye movements. Generally, both SCA-8 patients, who have normal life spans, and SCA-1 patients have very active reflexes. SCA-7 patients develop visual loss. In SCA types 1 to 3 and 7, there can be an earlier age of onset with increased severity (called anticipation) from one generation to the next. The size of the repeat expansion zone in the affected genes roughly correlates with the severity and age of onset. Penetrance is quite high; however, there are rare cases in which people do not develop symptoms. The reason for the lack of complete penetrance is currently unknown.
Data from C Mariotti, R Fancellu, S Di Donato. An overview of the patient with ataxia. J Neurol. 2005;252:511-518.
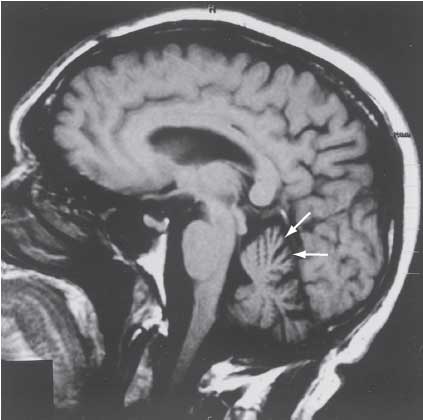
The diagnosis of SCA is initially suspected by the adult onset of symptoms. An MRI or computed tomography (CT) of the brain can detect atrophy (wasting) of the cerebellum and a variety of other subcortical structures (Figure 5–1). Genetic testing should be guided by clinical signs such as retinal degeneration, prominent involvement of noncerebellar symptoms, age of onset, eye-movement disorders, reduced stochastic (random pattern) velocity, and pyramidal signs as well as the patient’s ethnic origin/geographic location. The clinical features of the most common inherited ADCA disorders are listed in the accompanying table (see Table 5–1).
Figure 5–1. Sagittal MRI of the brain in spinocerebellar ataxia. (Reproduced, with permission, from Kasper DL, et al. Harrison’s Principles of Internal Medicine. 16th ed. New York, NY: McGraw-Hill; 2004: 2421.)
Once the genetic defect is characterized, family members can also be tested. These results must be interpreted cautiously, as there are intermediate ranges of repeats that show reduced penetrance, and age of onset and severity cannot be precisely predicted even in larger repeats. There are also cases of SCA diagnosed clinically that cannot be explained by any of the known genetic defects. It is estimated that in approximately 50% to 60% of Caucasian patients with a dominant familial form of cerebellar ataxia, DNA testing can provide a definitive diagnosis.
SCA-3 or Machado-Joseph disease (MJD) is the most common SCA subtype in most populations. The phenotype is one of the most variable among SCAs. The presenting syndromes for SCA-3 include pure cerebellar ataxia, familial parkinsonism, hereditary spastic paraplegia, hereditary neuropathy, and restless legs syndrome (RLS). A rarely recognized but common and rather specific sign of SCA-3 is impaired temperature discrimination in all limbs and even the trunk and face. Pseudoexophthalmos (bulging eyes caused by lid retraction), faciolingual myokymia, and dystonia have been thought to be characteristic, but not specific, signs of SCA-3.
SCA-3 MJD is an autosomal dominant inherited disorder with variable expression first described by Nakano and coworkers (1972) in an American family of Portuguese-Azorean descent. Since then more families with MJD have been reported worldwide. Three different clinical subtypes are described. Type I is characterized by an early age of onset (20-30 years), pyramidal and extrapyramidal signs, progressive external ophthalmoplegia (PEO), and minor cerebellar deficits. Type II involves an intermediate age of onset. Type III occurs after age 40 and includes ophthalmoparesis and anterior horn disease. At neuropathologic examination, degeneration of the cerebellum and the thoracic cord is always present in SCA-3, but degeneration of the striatum, substantia nigra, basis pons, oculomotor nuclei, and peripheral nerves is variable.
TREATMENT
There is currently no cure for ADCA and no treatment to slow the progression of the disease. Supportive treatment is important. Drugs that help control tremors are not effective for treating cerebellar tremors but they can be effective for parkinsonism, dystonia, RLS, and a variety of other neurologic symptoms. Physical therapy likely does not slow the progression of loss of coordination or muscle wasting, but affected patients should be encouraged to be active. Occupational therapy can be helpful in developing ways to accommodate the patient in performing daily activities. Walkers and other devices can assist the patient to have mobility. Other modifications such as ramps for a wheelchair, heavy eating utensils, and raised toilet seats can make patients more independent. Speech therapy and computer-based communication aids often help as the person loses his or her ability to speak.
Persons with SCA usually die one to two decades after symptoms develop. The prognosis for SCA-11 and SCA-6 is typically less severe, with a very slow worsening of symptoms, and persons with SCA-8 and SCA-11 have a normal life span.
COMPREHENSION QUESTIONS
5.1 A patient with SCA-3, in addition to having ataxia, has bradykinesia with rigidity and tremor at rest. Which of the following drugs is most likely to be helpful for the latter symptoms?
A. Carbidopa/levodopa
B. Haloperidol
C. Diazepam
D. Phenytoin
5.2 What radiologic feature is most characteristic of SCAs?
A. Cerebellar atrophy
B. High T2 signal in the cerebellar cortex
C. High signal lateral to the striatum
D. A high signal “hot cross bun” sign in the brainstem
5.3 Which of the following cases would be most suspicious for an ADCA?
A. Involvement of four of four siblings (two boys, two girls ages 4-12) and father in the same household affected with onset within 1 week of each other, but no other first- or second-degree relatives in a large kindred.
B. Male proband; affected proband’s father and one of two sisters and a paternal grandfather and uncle; unaffected, none of two brothers.
C. Male proband; neither parent affected; one of two brothers and one of two sisters in addition to a paternal great grandfather affected.
D. Male proband; parents, two brothers and two sisters unaffected; paternal grandfather and uncle affected.
ANSWERS
5.1 A. The tremor of SCA is often responsive to levodopa.
5.2 A. The cerebellum is most often affected. Optic nerve atrophy and spinal lesions may also be present in some SCAs. Answer B (high T2 signal in the cerebellar cortex) is characteristic of neoplastic cerebellar degeneration. Answer choices C (high signal lateral to the striatum) and D (high-signal “hot cross bun” sign in the brainstem) are seen with multisystem atrophy.
5.3 B. This option is typical for the autosomal dominant inheritance pattern;
answer choices C and D might be seen in a disease with poor penetrance. Answer choice A is suggestive of a toxic or infectious exposure.
CLINICAL PEARLS
|
▶ Hereditary SCAs commonly present in
adulthood clinically with cerebellar ataxia. Other neurologic signs may be
present but rarely with nonneurologic system involvement.
▶ The presence of an autosomal dominant
inheritance pattern is common, and the phenomenon of anticipation is common
in trinucleotide repeat diseases (each subsequent generation from the proband
presents earlier and with more severe disease manifestations).
▶ DNA testing can be diagnostic, but
clinical correlation is helpful in focused ordering of tests.
▶ Pharmacologic therapy does not alter
the natural course of cerebellar ataxia but can help to relieve neurologic
symptoms.
|
REFERENCES
Bataller L, Dalmau J. Paraneoplastic neurologic syndromes: approaches to diagnosis and treatment. Semin Neurol. 2003;23(2):215-224.
Bird TD. Hereditary ataxia overview. In: Pagon RA, Adam MP, Ardinger HH, et al., eds. GeneReviews® [Internet]. Seattle, WA: University of Washington; 1993-2016. http://www.ncbi.nlm.nih.gov/books/NBK1138/. Last accessed Nov 1, 2016.
Duen AM, Goold R, Giunti P. Molecular pathogenesis of spinocerebellar ataxias. Brain. 2006;129:
1357-1370.
Hadjivassiliou M, Grunewald R, Sharrack B, et al. Gluten ataxia in perspective: epidemiology, genetic susceptibility and clinical characteristics. Brain. 2003;126:685-691.
Harding AE. Hereditary spastic paraplegias. Semin Neurol. 1993;13:333-336.
Hekman KE, Gomez CM. The autosomal dominant spinocerebellar ataxias: emerging mechanistic themes suggest pervasive Purkinje cell vulnerability. J Neurol Neurosurg Psychiatry. 2015;86:554-561.
Løkkegaard T, Nielsen JE, Hasholt L, et al. Machado-Joseph disease in three Scandinavian families.
J Neurol Sci. 1998;156:152-157.
Mariotti C, Fancellu R, Di Donato S. An overview of the patient with ataxia. J Neurol. 2005;252:
511-518.
Schelhaasa HJ, Ippel PF, Beemerb FA, et al. Similarities and differences in the phenotype, genotype
and pathogenesis of different spinocerebellar ataxias. Eur J Neurol. 2000;7:309-314.
Schöls L, Bauer P, Schmidt T, et al. Autosomal dominant cerebellar ataxias: clinical features, genetics, and pathogenesis. Lancet Neurol. 2004;3:291-304.
Ruuskanen A, Kaukinen K, Collin P, et al. Gliadin antibodies in older population and neurological and psychiatric disorders. Acta Neurol Scand. 2013;127:19-25.
Tan EK, Ashizawa T. Genetic testing in spinocerebellar ataxias: defining a clinical role. Arch Neurol. 2001;58(2):191-195.

0 comments:
Post a Comment
Note: Only a member of this blog may post a comment.