Internal Medicine Pulmonary Embolism Case File
Eugene C. Toy, MD, Gabriel M. Aisenberg, MD
Case 14
A 48-year-old woman is brought to the emergency center complaining of a sudden onset of dyspnea. She reports that she was standing in the kitchen making dinner when she suddenly felt as if she could not get enough air. Also, her heart started racing, she became light-headed, and she felt as if she would faint. She denies chest pain or cough. Her medical history is significant only for a cholecystectomy performed 2 weeks earlier for gallstones. The procedure was complicated by a wound infection, requiring her to stay in the hospital for 8 days. She takes no medications regularly and only takes acetaminophen as needed for pain at her abdominal incision site.
On examination, she is tachypneic with a respiratory rate of 28 breaths/min, oxygen saturation of 84% on room air, heart rate of 124 beats per minute (bpm), and blood pressure of 118/89 mm Hg. She appears uncomfortable, diaphoretic, and frightened. Her oral mucosa is slightly cyanotic, her jugular venous pressure is elevated, and her chest is clear to auscultation. Her heart rhythm is tachycardic but regular with a loud second sound in the left second intercostal space, without gallop or murmurs. Her abdominal examination is benign, with a clean incision site without signs of infection. Her right leg is moderately swollen from her midthigh to her foot, and her thigh and calf are mildly tender to palpation. Laboratory studies, including cardiac enzymes, are normal; her electrocardiogram (ECG) reveals only sinus tachycardia, and her chest x-ray is interpreted as normal.
▶ What is the most likely diagnosis?
▶ What is the most appropriate diagnostic step?
▶ What are the common risk factors for this condition?
ANSWERS TO CASE 14:
Pulmonary Embolism
Summary: A 48-year-old woman presents with
- Recent surgery and hospitalization
- Acute onset of dyspnea
- Tachypnea, tachycardia, and hypoxemia
- Elevated jugular venous pressure and an accentuated pulmonic component of S2, suggestive of elevated pulmonary pressures
- A clear chest radiograph
Most likely diagnosis: Pulmonary embolism (PE) due to acute-onset dyspnea with history of recent hospitalization and immobilization.
Most appropriate diagnostic step: Chest computed tomography pulmonary angiogram (CTPA) with intravenous contrast or V/Q (ventilation/perfusion) scan.
Common risk factors: Recent surgery, immobilization, malignancy, pregnancy, certain medications (eg, oral contraceptives), and genetic factors.
- Understand the factors that predispose patients to develop thromboembolic disease. (EPA 12)
- Recognize the clinical presentation of PE. (EPA 1, 2)
- Describe the strategies to diagnose PE. (EPA 3)
- Understand the goals and methods of treatment of thromboembolism. (EPA 4, 10, 12)
Considerations
Pulmonary embolism is a difficult diagnosis to establish because of the nonspecificity of presenting signs and symptoms and the probabilistic nature of the most common noninvasive diagnostic tests. In patients with suspected PE, initial treatment is supportive to maintain adequate oxygenation and hemodynamic stability while efforts are undertaken to diagnose the cause of the patient’s symptoms. Often, a series of diagnostic tests is necessary to determine the likely diagnosis. Specific treatment of PE may include thrombolysis or surgical embolectomy for unstable patients and initiation of anticoagulation as a long-term measure to prevent recurrence.
APPROACH TO:
Pulmonary Embolism
DEFINITIONS
D-DIMER: A major fibrin degradation product that is released upon fibrinolysis. Elevated plasma levels of D-dimer indicate recent or ongoing intravascular coagulation and fibrinolysis.
DEEP VENOUS THROMBOSIS (DVT): Blood clot in the deep venous system that usually affects the lower extremities or pelvic veins.
LOW-MOLECULAR-WEIGHT HEPARIN (LMWH): A fragment of the larger mucopolysaccharide, heparin, that activates antithrombin III, inhibiting the final common pathway of the coagulation cascade.
PULMONARY EMBOLISM: A clot (usually originating from the lower extremity veins) that travels through the venous circulation and becomes lodged in the pulmonary artery or one of its branches. PE causes acute pulmonary hypertension and is labeled “massive PE” if it causes hemodynamic instability; it is labeled “submassive” or “moderate” if it causes right ventricular enlargement, strain, or dysfunction but is not associated with hemodynamic instability.
CLINICAL APPROACH
Epidemiology
Diagnosis and management of PE require a combination of clinical suspicion and appropriate use of diagnostic tools. Pulmonary emboli usually arise from DVTs and occasionally from less common sources, including air, fat, amniotic fluid, or tumor thrombus. More than 100 years ago, Rudolf Virchow postulated three factors that predispose to venous thrombus: local trauma to vessel wall, a state of hypercoagulability, and venous stasis. Genetic predisposition to hypercoagulability accounts for approximately 20% of PEs. The most common inherited conditions are the factor V Leiden mutation and the prothrombin gene mutations. Malignancy is also a predisposing condition for DVT. Neoplastic cells are thought to generate thrombin or to synthesize various procoagulants. Surgery and prolonged immobilization also increase the risk of PE up to 1 month postoperatively.
Pathophysiology
When venous thrombi dislodge from their site of formation, they may embolize to the pulmonary arteries, causing PEs. The deep proximal lower extremity veins are the most common sites of clot formation, although thromboses in pelvic, calf, and upper extremity veins may also embolize. Emboli to the pulmonary artery cause vascular obstruction and release of vasoactive agents such as serotonin, thereby elevating pulmonary vascular resistance. The resulting increase in alveolar dead space and subsequent redistribution of blood flow create areas of V/Q mismatch and impair gas exchange. Reflex bronchoconstriction increases airway resistance. This cascade can result in pulmonary edema, hemorrhage, or loss of surfactant, further decreasing lung compliance. As pulmonary vascular resistance increases, right heart wall tension rises, resulting in dilation and dysfunction that ultimately may impair left heart function. Progressive right heart failure is the usual cause of death from PE.
Clinical Presentation
Physical Examination. PE can often mimic other cardiopulmonary diseases, making the diagnosis challenging. Acute onset of dyspnea is the most common symptom of PE, and tachypnea is the most frequently observed sign. Severe dyspnea accompanied by syncope, hypotension, or cyanosis may indicate massive PE, whereas pleuritic pain, cough, or hemoptysis may suggest a smaller, more peripheral embolus causing infarction of lung tissue. Classic findings on physical examination include tachycardia and signs of right ventricular dysfunction, including increased jugular venous pressure, accentuated pulmonic component of the second heart sound, and systolic murmur that increases with inspiration. Findings suggestive of DVT include pain, swelling, and erythema of the lower extremity, particularly the back of the leg below the knee. Some patients complain of calf tenderness.
D-dimer Test. The most useful nonimaging diagnostic test is the serum D-dimer enzyme-linked immunosorbent assay (ELISA). It is elevated (> 500 ng/mL) in more than 95% of patients with PE, reflecting the breakdown of fibrin and thrombolysis. Although the D-dimer ELISA has a high negative predictive value and thus is useful in excluding PE, one should keep in mind that it lacks specificity if the pretest probability of PE is low. Elevations may be seen in patients with myocardial infarction, pneumonia, heart failure, cancer, or sepsis. Additional laboratory tests can assist in risk stratification of submassive PE, including brain natriuretic peptide (BNP) and cardiac troponins. Abnormalities on the ECG are less useful in the evaluation of PE. The most common finding is sinus tachycardia. The S1Q3T3 (S wave in lead I, a Q wave in lead III, and an inverted T wave in lead III, respectively) is often discussed but seen only in a minority of patients. When present, it is relatively specific.
Imaging Modalities. Radiologic studies are critical in the diagnosis of PE and DVT. A chest x-ray is the first study indicated in a symptomatic patient with new-onset dyspnea. A normal or near-normal chest x-ray is the most common finding in PE, sometimes with nonspecific abnormalities, such as atelectasis. In general, acute onset of hypoxemia in a patient with a normal chest x-ray should be interpreted as PE until otherwise proven. Classic abnormalities associated with PE include Westermark sign (decreased pulmonary vascularity distal to the clot), Hampton hump (peripheral wedge-shaped density above the diaphragm), and Palla sign (enlargement of the right descending pulmonary artery). The chest radiograph probably is more important in identifying other significant pulmonary parenchymal disease (pneumonia, pulmonary edema) and cardiac disease (cardiomyopathy) as alternative causes of the respiratory symptoms.
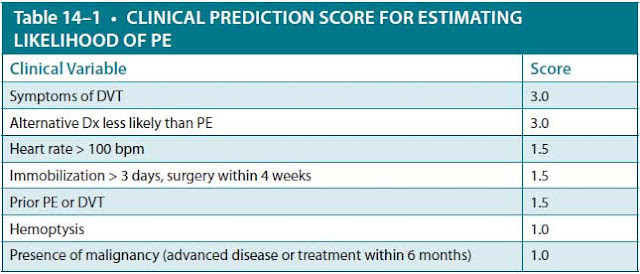
For any imaging modality, the most accurate diagnosis will be achieved in combination with the clinical suspicion. The Wells score is a useful clinical calculator to clinically estimate pretest probability of PE. A point score less than 4 with a negative D-dimer assay indicates a low probability for PE. A score
DX, diagnosis.
7 points or more = high probability for PE.
Less than 4 points, with negative D-dimer = low probability for PE.
Data from Wells PS, Anderson DR, Rodger M, et. al. Derivation of a simple clinical model to categorize patients’ probability of pulmonary embolism: increasing the models utility with the SimpliRED D-dimer. Thromb Haemost. 2000;83(3):416-420.
of 2 to 6 points indicates moderate probability, and more than 6 points is high probability (Table 14–1).
Chest computed tomography angiogram (CTA) with intravenous contrast is now the principal imaging modality to diagnose suspected PE. Current-generation spiral CT can acquire high-resolution images in a single breath hold and can visualize small branch artery emboli. In addition, the chest CT has the additional benefit of visualizing other abnormalities, such as pneumonia, aortic abnormalities, or pulmonary masses, that may not have been apparent on routine chest radiograph and may provide an alternative diagnosis for the patient’s symptomatology. The main caveats in the use of CT are the image quality and the experience of the center in interpreting this type of scan. In general, however, CT has been shown to be at least as accurate as the previously accepted standard imaging modality, V/Q lung scanning.
In patients in whom a CT with radiocontrast cannot be obtained or is contraindicated (such as in cases of advanced renal insufficiency or severe contrast allergy), a V/Q scan remains a useful tool. This study evaluates the circulation of air (distribution of inhaled xenon-133) and blood (distribution of technetium-99 aggregates with albumin) in the lungs. A segmental or lobar area with proper air distribution but with no perfusion is diagnostic of PE. A normal scan or a low-probability scan with a low clinical suspicion for PE effectively excludes the diagnosis.
If the CT and/or V/Q scan are nondiagnostic and yet the clinical suspicion remains high, other imaging modalities may be obtained. A lower extremity venous ultrasound demonstrating an acute DVT in a patient with signs and symptoms of PE would be sufficient to diagnose and treat PE (especially since the treatment with anticoagulation is the same). It should be noted, though, that a normal ultrasound does not exclude the diagnosis of PE since most patients with PE do not have evidence of residual DVT and since in many cases the clot has already embolized.
Figure 14–1. Diagnostic algorithm for patients with suspected pulmonary embolism. CT, computed tomography; ECHO, echocardiography; MR, magnetic resonance; PE, pulmonary embolism. (Adapted with permission, from Braunwald E, Fauci AS, Kasper KL, et al. Harrison’s Principles of Internal Medicine. 17th ed. 2008. Copyright © McGraw Hill LLC. All rights reserved.)
Other imaging studies, such as contrast-enhanced magnetic resonance imaging (MRI) or echocardiography (especially transesophageal echocardiography) may be used when the clinical suspicion remains high, but other diagnostic studies are inconclusive. Pulmonary artery angiography used to be the gold standard, but the methods described previously are as sensitive, specific, and less invasive; thus, it is no longer preferred. Figure 14–1 shows a diagnostic algorithm for suspected PE.
Treatment
Treatment options can be categorized in terms of primary and secondary therapy based on different management goals. Primary therapy consists of clot dissolution or thrombolysis (with tissue plasminogen activator [tPA]) or removal of the clot by surgical embolectomy. Primary therapy is usually reserved for patients with a high risk for adverse outcomes if the clot remains, that is, those with evidence of right heart failure or hemodynamic instability. The main criterion for thrombolytic administration is a systolic blood pressure < 90 mm Hg in the absence of absolute contraindications to tPA. The main complications of thrombolytic administration are bleeding, a very small percentage of which can be devastating intracerebral hemorrhages. Patients who have conventional surgical embolectomy performed emergently for “rescue” have a higher mortality (approaching 50%); however in less urgent circumstances, mortality rates are about 7%.
Newer therapies include half-dose thrombolytics and catheter-directed thrombolysis for submassive PE. Patients with hemodynamic collapse may require mechanical circulatory support with venoarterial extracorporeal membrane oxygenation (ECMO), which provides cardiac and pulmonary support, but this should only be employed in patients who are likely to improve with a definitive therapy (ie, surgical embolectomy).
For patients who are normotensive with normal RV function, the treatment is with anticoagulation, with the goal of secondary prevention of thrombus extension or recurrence. Anticoagulation does not dissolve an existing thrombus, but it allows for endothelialization and organization, which begins within days of treatment. Immediate anticoagulation should be initiated with intravenous unfractionated heparin (UFH), subcutaneous LMWH (eg, enoxaparin or tinzaparin), or the direct factor Xa inhibitor fondaparinux. While UFH requires a continuous infusion and frequent laboratory monitoring every 4 to 6 hours, LMWH and fondaparinux have similar efficacy and safety profiles. Both LMWH and fandaparinux provide rapid onset of action and predictable dose response, and laboratory monitoring is generally not required. Non–vitamin K antagonist oral anticoagulants (NOACs), which include direct thrombin inhibitors (dabigatran) and Xa inhibitors (rivaroxaban, apixaban and edoxaban), are now approved for the treatment of DVT and also do not require laboratory monitoring.
While patients are still on heparin, they can start therapy with the oral vitamin K antagonist warfarin. Because its biological effect is unpredictable, warfarin requires routine monitoring of the prothrombin time, standardized across laboratories as the international normalized ratio (INR). The target therapeutic INR is usually 2–3. When initiating warfarin therapy, the usual course is to use UFH, LMWH, or fondaparinux for at least 5 days while overlapping with warfarin (commonly referred to as “bridging”) until the INR has been therapeutic for 2 consecutive days. Rivaroxaban and apixaban have the added advantage that a bridging dose of heparin is not required.
The duration of treatment relates to the risk of recurrence. One factor in assessing this risk is whether the DVT or PE was provoked (ie, occurred due to a readily identifiable and transient event, eg, trauma or surgery) or unprovoked. For provoked DVT of the calf or upper extremity, 3 months of anticoagulation are recommended. Six months are recommended for patients with provoked proximal leg DVT or PE. For patients with idiopathic or unprovoked DVT or PE or with ongoing risk factors, such as malignancy or antiphospholipid syndrome, the duration of therapy is controversial, but indefinite anticoagulation may be required.
Inferior vena cava filter placement to prevent recurrent PE is recommended when there is active bleeding or other contraindication to anticoagulation or when there is recurrent DVT or PE despite therapeutic anticoagulation.
CASE CORRELATION
- See also Case 3 (Acute Coronary Syndrome), Case 5 (Aortic Dissection/Marfan Syndrome), Case 10 (Acute Pericarditis Caused by Systemic Lupus Erythematosus), Case 15 (Chronic Obstructive Pulmonary Disease), and Case 16 (Chronic Cough/Asthma).
COMPREHENSION QUESTIONS
14.1 A 35-year-old woman presents with calf tenderness and acute dyspnea. The arterial blood gas reveals a partial pressure of oxygen (Po2) of 76 mm Hg. Which of the following is the most common physical examination finding of PE?
A. Wheezing
B. Increased pulmonary component of the second heart sound
C. Tachypnea
D. Calf swelling
E. Pulmonary rales
14.2 A 39-year-old man is noted to have a DVT without any known risk factors. He notes that his brother also developed a PE at age 45, and his mother developed a “clot in the leg” when she was in her 30s. Which of the following is the most likely inherited disorder in this patient?
A. Protein S deficiency
B. Antithrombin III deficiency
C. Factor V Leiden mutation
D. Antiphospholipid antibody syndrome
E. Familial malignancy syndrome
14.3 A 54-year-old woman is being evaluated in the emergency center with shortness of breath of 12 hours’ duration. She also has significant vaginal bleeding of 1 month’s duration. On examination, she is found to have significant pallor of her sclera and skin. Speculum examination showed a large necrotic and exophytic mass of the cervix. The hemoglobin level is 7 g/dL. Her left leg is swollen and markedly different from her right leg. Doppler investigation reveals a DVT of the left leg. Which of the following is the best treatment for the thrombus?
A. Intravenous unfractionated heparin
B. Fractionated subcutaneous heparin
C. Subcutaneous unfractionated heparin
D. Oral warfarin (Coumadin)
E. Vena cava filter
ANSWERS
14.1 C. Tachypnea is the most common physical sign associated with PE. Calf or thigh pain and/or swelling (answer D) occurs less frequently than tachypnea. Other common clinical manifestations of pulmonary embolus in decreasing frequency include pleuritic pain, cough, and orthopnea. Wheezing (answer A), a sound caused by narrowing of the airway as seen in asthma or chronic obstructive pulmonary disease (COPD), can occur in PE but is less common. Rales (answer E) are rattling or crackling noises heard on auscultation of the lungs due to fluid or exudate in the alveoli. It is an uncommon sign in patients with PE. An increased pulmonary component of S2 (answer B) is also a possible sign in PE due to increased pressures in the pulmonary vasculature, but it is not the most common sign.
14.2 C. Factor V Leiden mutation is the most common hereditary thrombophilia. It is inherited in an autosomal dominant fashion and therefore will affect both men and women. The other answer choices (answer A, protein S deficiency; answer B, antithrombin III deficiency; answer D, antiphospholipid antibody syndrome; and answer E, familial malignancy syndrome) are all causes of hereditary thrombophilia with increased risk of PE but are less common causes.
14.3 E. This patient likely has cervical cancer with significant vaginal bleeding and anemia. This is a relative contraindication for anticoagulation (answers A [intravenous UFH]; answer B [fractionated subcutaneous heparin]; answer C [subcutaneous UFH]; and answer D [oral warfarin]) since these agents would exacerbate the bleeding. Thus, a vena cava filter is the most appropriate choice in this patient; the filter ideally prevents thrombi from traveling to the lungs.
CLINICAL PEARLS
▶ Acute onset of dyspnea or hypoxemia with a normal chest x-ray should be considered caused by a PE until proven otherwise.
▶Diagnosis of PE is usually established using imaging tests such as chest CT pulmonary angiogram in light of clinical pretest probability.
▶The clinical suspicion guides the pursuit of diagnosis of thromboemboli.
▶The primary therapy of DVT or PE is anticoagulation, with the goal of pre-venting recurrence.
REFERENCES
Becattini C, Agnelli G. Treatment of venous thromboembolism with new anticoagulant agents. J Am Coll Cardiol. 2016;67(16):1941-1955.
Elliot CG. Pulmonary physiology during pulmonary embolism. Chest. 1992;101:89S-185S.
Goldhaber SZ. Deep venous thrombosis and pulmonary thromboembolism. In: Jameson JL, Fauci AS,
Kasper DL, et al, eds. Harrison’s Principles of Internal Medicine. 20th ed. New York, NY: McGraw Hill; 2018:2170-2177.
Jaff MR, McMurty MS, Archer SL, et al. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension. Circulation. 2011;123:1788-1830.
Lip GYH, Hull RD. Venous thromboembolism: initiation of anticoagulation (first 10 days).
Leung LLK, Mandel J, ed. UpToDate. Waltham, MA: UpToDate Inc. https://www.uptodate.com/contents/venous-thromboembolism-initiation-of-anticoagulation-first-10-days. Accessed June 8, 2019.
Sharifi M, Bay C, Skrocki L, et al. Moderate pulmonary embolism treated with thrombolysis
(the MOPETT Trial). Am J Cardiol. 2013;111:273-277.
Solari F, Varacallo M. Low molecular weight heparin (LMWH) [Updated 2019 Feb 1]. In:
StatPearls [Internet]. Treasure Island, FL: StatPearls; 2019. https://www.ncbi.nlm.nih.gov/books/NBK525957/. Accessed March 27, 2020
Van Belle A, Buller HR, Huisman MV, et al. Effectiveness of managing suspected pulmonary embolism using an algorithm combining clinical probability, D-dimer testing, and computed tomography. JAMA. 2006;295:172-179.
Wells PS, Anderson DR, Rodger M, et al. Derivation of a simple clinical model to categorize patients’ probability of pulmonary embolism: increasing the models utility with the SimpliRED D-dimer. Thromb Haemost. 2000;83(3):416-420.
Zehnder JL. Clinical Use of Coagulation Tests. Leung LLK, ed. UpToDate. Waltham, MA: UpToDate Inc. https://www.uptodate.com/contents/clinical-use-of-coagulation-tests. Accessed June 8, 2019.


0 comments:
Post a Comment
Note: Only a member of this blog may post a comment.