Internal Medicine Acute Kidney Injury Case File
Eugene C. Toy, MD, Gabriel M. Aisenberg, MD
Case 30
A 54-year-old man with a history of type 2 diabetes mellitus and coronary artery disease is admitted to the coronary care unit with worsening angina and hypertension. His pain is controlled with intravenous nitroglycerin, and he is treated with aspirin, beta-blockers to lower his heart rate, and angiotensinconverting enzyme (ACE) inhibitors to lower his blood pressure. Cardiac enzymes are normal. He undergoes coronary angiography, which reveals no significant stenosis. By the next day, his urine output has diminished to 200 mL over 24 hours. Examination at that time reveals that he is afebrile with heart rate of 56 beats per minute (bpm) and blood pressure 109/65 mm Hg. His neck veins are flat, chest is clear, and heart rhythm is normal with an S4 gallop and no murmur or friction rub. His abdomen is soft without masses or bruits. He has no peripheral edema or rashes, with normal pulses in all extremities. His fundoscopic examination reveals dot hemorrhages and hard exudates. Current laboratory studies include Na 140 mEq/L, K 5.3 mEq/L, Cl 104 mEq/L, CO2 19 mEq/L, and blood urea nitrogen (BUN) 69 mg/dL. His creatinine (Cr) level has risen to 2.9 mg/dL from 1.6 mg/dL on admission.
▶ What is the patient’s new clinical problem?
▶ What is the strongest risk factor for this condition?
▶ What might have prevented this condition?
▶ What is your next diagnostic step?
ANSWERS TO CASE 30:
Acute Kidney Injury
Summary: A 54-year-old man presents with
- Coronary artery disease and diabetes mellitus type 2 with retinopathy
- Angina and hypertension, for which he is receiving oral aspirin, beta-blockers, an ACE inhibitor, and nitroglycerin
- No significant stenosis upon coronary angiography using contrast dye
- Currently normotensive state with likely diabetic nephropathy
- Creatinine increased to 2.9 mg/dL from 1.6 mg/dL on admission
- Oliguria
New clinical problem: Acute kidney injury (AKI) evidenced by increased creatinine from baseline values and oliguria.
Strongest risk factor: Existing kidney disease—elevated baseline creatinine.
Possible prevention: Intravenous hydration with normal saline prior to angiography.
Next diagnostic step: Urinalysis and urine chemistries to determine whether the process is prerenal, intrinsic renal, or less likely postrenal.
ANALYSIS
Objectives
- Identify the common causes, evaluation, and prevention of AKI in hospitalized patients. (EPA 1, 2, 3)
- Appraise urinalysis and serum chemistry values in the diagnostic approach of AKI to be able to categorize the etiology as prerenal, renal, or postrenal. (EPA 3)
- Discuss the management of hyperkalemia and indications for acute dialysis. (EPA 4, 9, 10)
Considerations
A 54-year-old man with diabetes, retinopathy, and some chronic kidney disease develops AKI in the hospital, as indicated by the elevated serum creatinine level of 2.9 mg/dL and BUN of 69 mg/dL. He has undergone several medical therapies and procedures, all of which might be potentially contributory: acute lowering of his blood pressure, ACE inhibitor, iodinated radiocontrast media, and arterial catheterization with possible atheroemboli. The mortality rate associated with critically ill patients who develop AKI is high; thus, identifying and treating the underlying etiology of this patient’s kidney failure and taking measures to protect the kidneys from further damage are essential.
APPROACH TO:
Acute Kidney Injury
DEFINITIONS
ACUTE KIDNEY INJURY: Abrupt decline in kidney function, measured as decreased glomerular filtration rate (GFR). True GFR is difficult to measure, so we rely on increases in serum creatinine levels or decreases in urine production to indicate a fall in GFR.
ANURIA: Less than 50 mL of urine output in 24 hours. Acute obstruction, cortical necrosis, and vascular catastrophes, such as aortic dissection, should be considered in the differential diagnosis.
OLIGURIA: Less than 400 mL of urine output in 24 hours. Physiologically, it is the lowest amount of urine a person on a normal diet can make if he or she is severely dehydrated and does not retain uremic waste products. Oliguria is a poor prognostic sign in AKI. Patients with oliguric renal failure have higher mortality rates and worse renal recovery than patients who are nonoliguric.
UREMIA: Nonspecific symptoms of fatigue, weakness, nausea with early morning vomiting, itchiness, confusion, pericarditis, and coma attributed to the retention of waste products in renal failure but do not always correlate with the BUN level. A highly malnourished patient with renal failure may have a modestly elevated BUN and be uremic. Another patient may have a highly elevated BUN and be asymptomatic. Elevated BUN without symptoms is called azotemia.
CLINICAL APPROACH
Pathophysiology
The differential diagnosis of AKI proceeds from consideration of three basic pathophysiologic mechanisms: prerenal failure, postrenal failure, and intrinsic renal failure.
Individuals with prerenal failure experience diminished GFR as a result of a marked decreased renal blood perfusion so that less glomerular filtrate is formed. Sometimes, the clinical presentation is straightforward, such as volume depletion from gastrointestinal fluid loss or hemorrhage; other times, the presentation of patients with prerenal failure can be more confusing. For example, a patient with severe nephrotic syndrome may appear to be volume overloaded because of the massive peripheral edema present; however, the effective arterial blood volume is very low as a consequence of severe hypoalbuminemia, resulting in a prerenal AKI. Similarly, a patient with severe congestive heart failure may have prerenal failure because of a low cardiac ejection fraction, yet at the same time be fluid overloaded with peripheral and pulmonary edema. The key is to assess “what the kidneys see” versus the remainder of the body. Typically, the BUN:Cr ratio is greater than 20 in prerenal failure. Medications such as aspirin, nonsteroidal anti-inflammatory drugs, and ACE inhibitors can alter intrarenal blood flow and result in prerenal failure. Table 30–1 provides an abbreviated listing of the etiologies of prerenal failure.
Postrenal failure, also referred to as obstructive nephropathy, implies blockage of urinary flow. The site of obstruction can be anywhere along the urinary system, including the intratubular region (crystals), ureters (stones, extrinsic compression by tumor), bladder, or urethra. By far, the most common causes of obstructive nephropathy are ureteral obstruction due to malignancy or urethral obstruction due to benign or malignant prostatic hypertrophy. The patient’s symptoms depend on whether or not both kidneys are involved, the degree of obstruction, and the time course of the blockage. This is usually diagnosed by observing hydronephrosis or bladder distension on ultrasound.
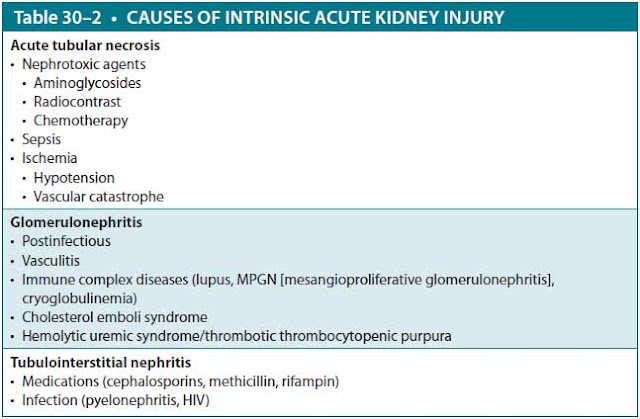
Intrinsic renal failure is caused by disorders that injure the renal glomeruli or tubules directly. These include glomerulonephritis, tubulointerstitial nephritis, and acute tubular necrosis (ATN) from ischemia, sepsis, or nephrotoxic agents. Table 30–2 lists major causes of intrinsic AKI.
Clinical Presentation
A thorough history and physical examination are important. Questions that are important to ask include
- Does the patient have signs or symptoms of a systemic disease, such as heart failure or cirrhosis, that could cause prerenal failure?
- Does the patient have symptoms of a disease, such as lupus, that could cause a glomerulonephritis?
- Did the patient receive something in the hospital that could cause ATN, such as intravenous contrast or an aminoglycoside?
- Did the patient present with severe infection and systemic inflammatory response syndrome that could lead to septic ATN?
- While in the operating room, did the patient become hypotensive from hemorrhage causing ischemic ATN?
- Is the patient receiving an antibiotic causing allergic interstitial nephritis?
In addition to the history and physical examination, urinalysis and measurement of urinary electrolytes are helpful in making the diagnosis.
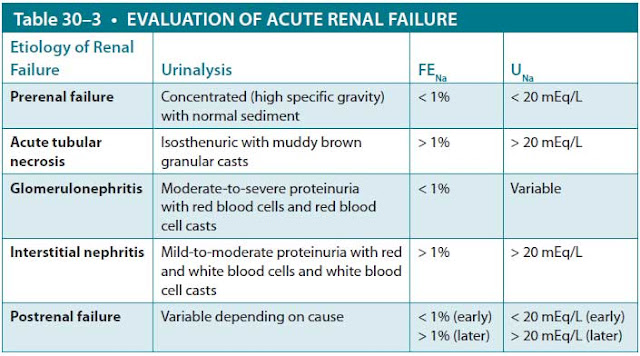
Urinalysis. The urine findings based on testing with reagent paper and microscopic examination help with the diagnosis of AKI (Table 30–3). In prerenal failure, urinalysis usually reveals a high specific gravity and normal microscopic findings. Individuals with postrenal failure are typically unable to concentrate the urine, so the urine osmolality is equal to the serum osmolality (isosthenuria), and the specific gravity is 1.010. The microscopic findings vary depending on the cause of the obstruction: hematuria (crystals or stones), leukocytes (prostatic hypertrophy), or normal (extrinsic ureteral compression from a tumor). Urinalysis of various intrinsic renal disorders may be helpful. Ischemic, septic, and nephrotoxic ATN are usually associated with urine that is isosthenuric, often with proteinuria, and containing “muddy brown” granular casts on microscopy. In glomerulonephritis, the urine generally reveals moderate-to-severe proteinuria, sometimes in the nephrotic range, and microscopic hematuria and red blood cell casts. Tubulointerstitial nephritis classically produces urine that is isosthenuric (the tubules are unable to concentrate the urine) with mild proteinuria. On microscopy, it usually reveals leukocytes, white cell casts, and sometimes urinary eosinophils.
Abbreviations: FENa, fractional excretion of sodium; UNa, urinary concentration of sodium.
NOTE: Though it remains widely used, the reliability of FENa to assess renal failure is poor since its conclusions arise from a very small study. It is important to highlight that, if there is suspicion for hypovolemia, rather than measuring FENa, it is more practical to assess the patient’s response to fluid resuscitation.
Urinary Electrolytes. Calculation of the fractional excretion of sodium (FENa) was devised to differentiate oliguric prerenal failure from oliguric ATN; it is of little use in other circumstances. FENa represents the amount of sodium filtered by the kidneys that is not reabsorbed. The kidneys of a healthy person on a normal diet typically reabsorb more than 99% of the sodium that is filtered, with a corresponding FENa less than 1%. Normally, the excreted sodium represents the dietary intake of sodium as a result of the maintenance of sodium homeostasis. In prerenal failure, decreased renal perfusion leads to a diminished GFR; if the renal tubular function is intact, FENa remains less than 1%. Furthermore, because the patient has either true volume depletion or “effective” volume depletion, serum aldosterone will stimulate the kidneys to retain sodium, and the urinary sodium will be low (< 20 mEq/L). On the other hand, in oliguric ATN, the renal failure is caused by tubular injury. Hence, there is tubular dysfunction with an associated inability to reabsorb sodium, leading to a FENa greater than 2% and a urinary sodium exceeding 20 mEq/L.
Measurements of FENa and urinary sodium are less helpful in other circumstances. For example, in nonoliguric ATN, the injury is usually less severe, so the kidneys may maintain normal sodium reabsorption and a FENa less than 1%. Diuretic medications, which interfere with sodium reabsorption, are often used for congestive heart failure or nephrotic syndrome. Although these patients may have prerenal failure, the use of diuretics will increase the urinary sodium and FENa. In acute glomerulonephritis, the kidneys often avidly resorb sodium, leading to very low urinary sodium levels and FENa. Early in the course of postobstructive renal failure caused by ureteral obstruction, the afferent arteriole typically undergoes intense vasoconstriction, with consequent low urinary sodium levels (Table 30–3).
Treatment
AKI is managed by identifying and reversing the underlying cause. Intravenous isotonic fluids are a mainstay of treatment for any prerenal AKI caused by true volume depletion. Diuretics, on the other hand, would be used to treat prerenal AKI in the setting of venous congestion in heart failure. Other common interventions include removing nephrotoxic medications, optimizing blood pressure and electrolytes, and administering diuretics. If the AKI is severe, the patient may require urgent hemodialysis.
The indications for dialysis in AKI include fluid overload (such as pulmonary edema), metabolic acidosis, hyperkalemia, uremic pericarditis, severe hyperphosphatemia, and uremic symptoms refractory to medical treatment. Because of the risk of fatal cardiac arrhythmias, severe hyperkalemia is considered an emergency best treated acutely medically, not with dialysis.
An urgent electrocardiogram should be performed on any patient with suspected hyperkalemia. If the classic peaked or “tented” T waves are present, intravenous calcium should be administered immediately. Although it will not lower the serum potassium level, the calcium will oppose the membrane effects of the high potassium concentration on the heart, allowing time for other interventions to lower the potassium level. One of the most effective methods for treating hyperkalemia is administration of intravenous insulin (usually 10 units), along with 50 to 100 mL of 50% glucose solution to prevent hypoglycemia. Insulin drives potassium into cells, lowering levels within 30 minutes. Potassium can also be driven intracellularly with a beta-agonist nebulizer, such as albuterol. In the presence of a severe metabolic acidosis, administration of intravenous sodium bicarbonate also promotes an intracellular shift of potassium, albeit less effectively. All three therapies have only a transient effect of lowering serum potassium levels because the total body potassium balance is unchanged, and the potassium eventually leaks back out of the cells. Definitive treatment of hyperkalemia, removal of potassium from the body, is accomplished by one of three methods: (1) administration of a loop diuretic (eg, furosemide) to increase urinary flow and excretion of potassium; (2) administration of potassium-binding cationic exchange resin (eg, sodium polystyrene sulfonate, patiromer, or sodium zirconium cyclosilicate) to prevent gastrointestinal absorption of potassium; or, finally, (3) dialysis.
Dose adjustment of medications that are excreted through the kidneys is necessary to prevent dose-related toxicity, even if the toxicity affects other organs.
CASE CORRELATION
- See also Case 28 (Acute Glomerulonephritis) and Case 29 (Nephrotic Syndrome and Diabetic Nephropathy).
COMPREHENSION QUESTIONS
30.1 A 63-year-old woman with a history of cervical cancer treated with a hysterectomy and pelvic irradiation now presents with acute oliguric renal failure. On physical examination, she has normal jugular venous pressure, is normotensive without orthostasis, and has a benign abdominal examination. Her urinalysis shows a specific gravity of 1.010, with no cells or casts on microscopy. Urinary FENa is 2%, and the Na level is 35 mEq/L. Which of the following is the best next step?
A. Bolus of intravenous fluids
B. Renal ultrasound
C. Computed tomographic scan of the abdomen with intravenous contrast
D. Administration of furosemide to increase her urine output
30.2 A 49-year-old man with a long-standing history of chronic renal failure as a consequence of diabetic nephropathy is brought to the emergency room for nausea, lethargy, and confusion. His physical examination is significant for an elevated jugular venous pressure, clear lung fields, and harsh systolic and diastolic sounds heard over the precordium. Serum chemistries reveal K 5.1 mEq/L, CO2 17 mEq/L, BUN 145 mg/dL, and creatinine 9.8 mg/dL. Which of the following is the most appropriate next step in therapy?
A. Administer intravenous insulin and glucose
B. Administer intravenous sodium bicarbonate
C. Administer intravenous furosemide
D. Begin hemodialysis urgently
30.3 A 62-year-old diabetic man underwent an abdominal aortic aneurysm repair 2 days ago. He is being treated with gentamicin for a urinary tract infection. His urine output has fallen to 300 mL over 24 hours, and his serum creatinine has risen from 1.1 mg/dL on admission to 1.9 mg/dL. Which of the following laboratory values would be most consistent with a prerenal etiology of his renal insufficiency?
A. FENa of 3%
B. Urinary sodium level of 10 mEq/L
C. Central venous pressure reading of 10 mm Hg
D. Gentamicin trough level of 4 μg/mL
ANSWERS
30.1 B. Renal ultrasound is the next appropriate step to assess for hydronephrosis and to evaluate for bilateral ureteral obstructions, which are common sites of metastases of cervical cancer. Her physical examination and urine studies (showing a FE > 1%) are inconsistent with hypovolemia, so intravenous infusion (answer A) is unlikely to improve her renal function. Use of loop diuretics (answer D) may increase her urine output somewhat but does not help to diagnose the cause of her renal failure or to improve her outcome. Further imaging (answer C) may be necessary after the ultrasound, but use of intravenous contrast at this point may actually worsen her renal failure.
30.2 D. The patient has uremia, hyperkalemia, and (likely) uremic pericarditis (harsh systolic and diastolic sounds of friction rub), which may progress to life-threatening cardiac tamponade unless the underlying renal failure is treated with dialysis. If the threat of tamponade is significant, pericardiocentesis may need to be performed prior to dialysis. As for the other treatments, insulin plus glucose (answer A) would treat hyperkalemia, and bicarbonate (answer B) would help with both metabolic acidosis and hyperkalemia, but this patient’s potassium and bicarbonate levels are only mildly abnormal and are not immediately life threatening. Furosemide (answer C) will not help because the patient does not have pulmonary edema; additionally, due to the renal insufficiency, the furosemide likely would not lead to much potassium excretion.
30.3 B. This elderly man has findings of renal insufficiency with two possible causes: prerenal due to volume depletion and acute kidney injury due to gentamicin/hypotension. The patient’s urinary sodium of 10 mEq/L points to a prerenal cause. Prerenal insufficiency connotes insufficient blood volume, typically with FENa less than 1% (not answer A, 3%) and urinary sodium less than 20 mEq/L. Supporting data would include a low central venous pressure reading (normal central venous pressure is 4-8 mm Hg), not an elevated reading of 10 mm Hg (answer C). The gentamicin level of 4 μg/mL is elevated (normal < 2 μg/mL) and may predispose to kidney damage, but intrarenally, thus ruling out answer D.
CLINICAL PEARLS
▶ The two main causes of AKI in hospitalized patients are prerenal azotemia from volume depletion and ATN.
▶ In the acutely anuric patient, one must quickly determine if the kidneys are obstructed or if the vascular supply is interrupted.
▶ Treatment of prerenal failure is volume replacement unless the cause is severe systolic heart failure; treatment of postrenal failure is relief of the obstruction.
▶ The main causes of postrenal failure are obstruction caused by prostatic hypertrophy in men and bilateral ureteral obstruction caused by abdominal or pelvic malignancy in either gender.
▶ Uremic pericarditis is an indication for urgent hemodialysis. Other indications include hyperkalemia, metabolic acidosis, severe hyperphosphatemia, and volume overload when refractory to medical management.
▶ Treatment of hyperkalemia can be remembered by the mnemonic C BIG K (calcium, bicarbonate/beta-agonist, insulin, glucose, Kayexalate [polystyrene sulfonate]).
▶ Hyperkalemia is treated initially with calcium to stabilize cardiac membranes (if there are electrocardiographic abnormalities); insulin and beta-agonists to redistribute potassium intracellularly (sodium bicarbonate if there is a severe metabolic acidosis); and then loop diuretics, a potassium exchange resin, or hemodialysis to remove excess potassium from the body.
▶ Indications for dialysis can be remembered by the mnemonic AEIOU (acidosis, electrolyte disturbances, ingestions of toxins, overload, uremia).
REFERENCES
Lameire N, Van Biesen W, Vanholder R. Acute renal failure. Lancet. 2005;365:417-430.
Rose BD, Post TW. Hyperkalemia. In: Clinical Physiology of Acid-Base and Electrolyte Disorders. 5th ed. New York, NY: McGraw Hill; 2001:913-919.
Waikar SS, Bonventre JV. Acute kidney injury. In: Jameson J, Fauci AS, Kasper DL, Hauser SL, Longo DL, Loscalzo J, eds. Harrison’s Principles of Internal Medicine. 20th ed. New York, NY: McGraw Hill; 2018.


0 comments:
Post a Comment
Note: Only a member of this blog may post a comment.