Internal Medicine Acute Coronary Syndrome Case File
Eugene C. Toy, MD, Gabriel M. Aisenberg, MD
Case 3
A 56-year-old man with a history of hypercholesterolemia and a 40-pack-year smoking history comes to the emergency department complaining of chest discomfort. He describes the discomfort as a severe, retrosternal pressure that woke him from sleep 3 hours earlier. On examination, he appears uncomfortable and diaphoretic, with a heart rate of 116 beats per minute (bpm), blood pressure of 166/102 mm Hg, respiratory rate of 22 breaths per minute, and oxygen saturation of 96% on room air. Jugular venous pressure appears normal. Auscultation of the chest reveals clear lung fields, a regular rhythm with an S4 gallop, and no murmurs or rubs. A chest radiograph shows clear lungs and a normal cardiac silhouette. The electrocardiogram (ECG) is shown in Figure 3–1.
Figure 3–1. Electrocardiogram. (Reproduced with permission, from Braunwald E, Fauci AS, Kasper KL,
et al. Harrison’s Principles of Internal Medicine. 16th ed. 2005. Copyright © McGraw Hill LLC. All rights
reserved.)
▶ What is the most likely diagnosis?
▶ What is the next step in therapy?
ANSWERS TO CASE 3:
Acute Coronary Syndrome
Summary: A 56-year-old man presents with
- Cardiovascular risk factors (hypertension and tobacco use)
- Acute onset of retrosternal pressure, tachycardia, hypertension, and diaphoresis
- S4 on cardiac auscultation, reflecting a stiff left ventricle (LV), which may result from ischemic myocardium
- ECG showing ST elevations and T-wave changes
Most likely diagnosis: Acute ST-segment elevation myocardial infarction (MI) (STEMI).
Next step in therapy: Administer aspirin and a beta-blocker and assess whether he is a candidate for rapid reperfusion of the myocardium, that is, treatment with thrombolytics or percutaneous coronary intervention.
- List the diagnostic criteria for acute MI. (EPA 3, 10)
- Identify patients who benefit from thrombolytics or percutaneous coronary intervention. (EPA 4, 10)
- Describe the complications of MI and their treatment options. (EPA 4, 10)
- Describe post-MI risk stratification and secondary prevention strategies. (EPA 12)
Considerations
The three most important issues for this patient are (1) the suspicion of acute MI based on the clinical and ECG findings, (2) deciding whether the patient has indications or contraindications for thrombolytics or primary percutaneous coronary intervention, and (3) excluding other diagnoses that might mimic acute MI but would not benefit from or might be worsened by anticoagulation or thrombolysis (eg, acute pericarditis, aortic dissection).
APPROACH TO:
Suspected Acute MI
DEFINITIONS
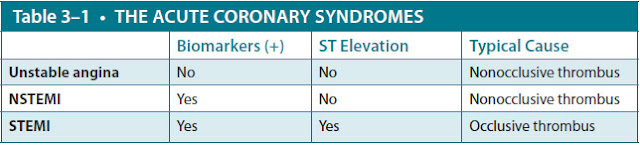
ACUTE CORONARY SYNDROME (ACS): An umbrella term for conditions of acute cardiac ischemia, usually caused by formation of a thrombus in a coronary artery. ACS is subdivided into three separate conditions: unstable angina, NSTEMI (non–ST-segment elevation myocardial infarction), and STEMI. These three conditions are distinguished from each other by biomarkers (troponins, CK-MB [creatine kinase myocardial band]) and ECG changes.
NON–ST-SEGMENT ELEVATION MYOCARDIAL INFARCTION (NSTEMI): Acute cardiac ischemia with death of cardiac myocytes. Death of myocytes results in intracellular proteins like troponins being leaked into the blood. Thus, NSTEMI is characterized by presence of troponins without ST elevation on ECG.
ST-SEGMENT ELEVATION MYOCARDIAL INFARCTION (STEMI): Acute cardiac ischemia with transmural cardiac infarction, resulting in ST-segment elevations of more than 0.1 mV in two or more contiguous leads AND elevated cardiac biomarkers. STEMI is the most severe form of ACS and is more likely to cause cardiogenic shock acutely.
THROMBOLYTICS: Drugs such as tissue plasminogen activator (tPA), streptokinase, and reteplase (recombinant plasminogen activator [r-PA]), which act to lyse fibrin thrombi in order to restore patency of the coronary artery when percutaneous coronary intervention (PCI) is contraindicated or is not available.
UNSTABLE ANGINA: Acute cardiac ischemia without death of cardiac myocytes. In unstable angina, a typical patient is experiencing chest pain but without serum troponin elevation and without ST elevation on ECG.
CLINICAL APPROACH
Pathophysiology
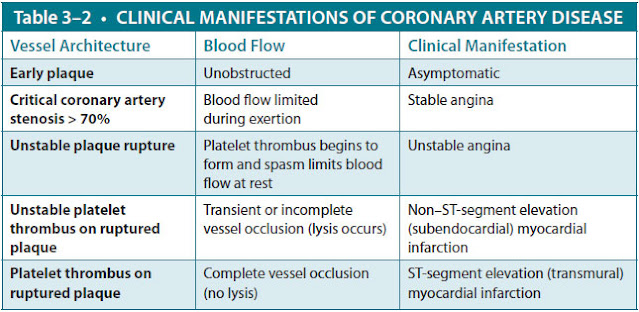
Acute coronary syndromes, which exist on a continuum ranging from unstable angina pectoris to NSTEMI to STEMI, are usually caused by in situ thrombosis at the site of a ruptured atherosclerotic plaque in a coronary artery. Occasionally, they are caused by embolic occlusion, coronary vasospasm, vasculitis, aortic root or coronary artery dissection, or cocaine use (which promotes both vasospasm and thrombosis). The resultant clinical syndrome is related to both the degree of atherosclerotic stenosis in the artery and the duration and extent of sudden thrombotic occlusion of the artery. If the occlusion is incomplete or if the thrombus undergoes spontaneous lysis, unstable angina occurs. If the occlusion is complete for longer than 30 minutes, infarction occurs. In contrast, the mechanism of chronic stable angina is a flow-limiting stenosis usually caused by atherosclerotic plaque that causes ischemia during exercise without acute thrombosis (Table 3–1).
Clinical Presentation
Signs and Symptoms. Chest pain is the cardinal feature of MI, even though it is not universally present (Table 3–2). It is similar to angina pectoris—described as heavy, squeezing, or crushing—and is localized to the retrosternal area or epigastrium, sometimes radiating to the arm, lower jaw, or neck. Unlike stable angina, however, it persists for more than 30 minutes and is not relieved by rest. The pain often is accompanied by sweating, nausea, vomiting, and/or the sense of impending doom. In some patients, chest pain may not be prominent. Diabetics and older patients may present with only vague discomfort or have sudden dyspnea, pulmonary edema, or ventricular arrhythmias as their initial presentation. There are no specific physical findings in a patient with an acute MI. Many patients are anxious and diaphoretic. Cardiac auscultation may reveal an S4 gallop, reflecting myocardial noncompliance because of ischemia; an S3 gallop, representing severe systolic dysfunction; or a new apical systolic murmur of mitral regurgitation caused by ischemic papillary muscle dysfunction.
Electrocardiogram. The ECG is often critical in diagnosing an acute MI and guiding therapy. A series of ECG changes reflects the evolution of the infarction (Figure 3–2).
- The earliest changes are tall, positive, hyperacute T waves in the ischemic vascular territory.
- This is followed by elevation of the ST segments (myocardial “injury pattern”).
- Over hours to days, T-wave inversion frequently develops.
- Finally, diminished R-wave amplitude or Q waves occurs, representing significant myocardial necrosis and replacement by scar tissue.
When acute ischemia is limited to the subendocardium, ST-segment depression, rather than ST-segment elevation, develops. ST-segment elevation indicates that the full thickness of the wall has been affected (ie, “transmural” infarction).
Figure 3–2. Temporal evolution of ECG changes in acute MI. Note tall hyperacute T waves and loss of R-wave amplitude, followed by ST-segment elevation, T-wave inversion, and development of Q waves. Persistent ST-segment elevation suggests LV aneurysm. (Reproduced with permission, from Alpert JS. Cardiology for the Primary Care Physician. 2nd ed. 1998. Copyright © Appleton & Lange. All rights reserved.)
From the ECG we can localize the ischemia related to a vascular territory supplied by one of the three major coronary arteries. STEMI is defined as ST-segment elevation more than 0.1 mV in two or more contiguous leads (ie, in the same vascular territory) and/or a new left bundle branch block (LBBB) (which obscures usual ST-segment analysis). As a general rule, leads II, III, and aVF correspond to the inferior surface of the heart supplied by the right coronary artery (RCA); leads V2 to V4 correspond to the anterior surface supplied by the left anterior descending coronary artery (LAD); and leads I, aVL, V5, and V6 correspond to the lateral surface, supplied by the left circumflex coronary artery (LCX).
Cardiac Biomarkers. Certain proteins, referred to as cardiac biomarkers, are released into blood from necrotic heart muscle after an acute MI. The creatine phosphokinase (CK) level rises within 4 to 8 hours and returns to normal by 48 to 72 hours. Creatine phosphokinase is found in skeletal muscle and other tissues, but the CK-MB isoenzyme is not found in significant amounts outside of heart muscle, so elevation of this fraction is more specific for myocardial injury. Cardiac-specific troponin I (cTnI) and cardiac-specific troponin T (cTnT) are more specific to heart muscle and are the preferred markers of myocardial injury. These protein levels rise approximately from 3 to 5 hours after infarct. The cTnI levels may remain elevated for 7 to 10 days and cTnT levels for 10 to 14 days. They are very sensitive and fairly specific indicators of myocardial injury, and their levels may be elevated with even small amounts of myocardial necrosis. Generally, two sets of normal troponin levels 6 to 8 hours apart exclude MI.
Differential Diagnosis. The diagnosis of acute MI is made by finding at least two of the following three features: typical chest pain persisting for more than 30 minutes, typical ECG findings, and elevated cardiac biomarker levels. Because of the urgency in initiating treatment, diagnosis often rests on the clinical history and the ECG findings while determination of cardiac biomarker levels is pending. During the initial evaluation, other conditions that present with chest pain but could impair with the treatment of ACS should be excluded. Two such conditions are aortic dissection and acute pericarditis. Aortic dissection often presents with unequal pulses or blood pressures in the arms, a new murmur of aortic insufficiency, or a widened mediastinum on chest x-ray film. Acute pericarditis often presents with chest pain and a pericardial friction rub, but the ECG findings show diffuse ST-segment elevation rather than those limited to a vascular territory.
Treatment
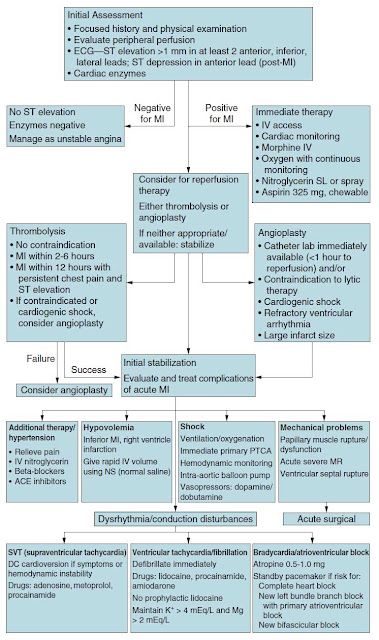
Once an acute MI has been diagnosed based on history, ECG, or cardiac biomarkers, several therapies are initiated. Because the process is caused by acute thrombosis, antiplatelet agents such as aspirin and anticoagulation with heparin are used. To limit infarct size, beta-blockers are used to decrease myocardial oxygen demand, and nitrates are given to increase coronary blood flow. All of these therapies appear to reduce mortality in patients with acute MI. In addition, morphine may be given to reduce pain and the consequent tachycardia. Patients are also placed on supplemental oxygen (Figure 3–3). After this medical management, reperfusion therapy should be considered. The major options are thrombolytics or PCI.
Percutaneous coronary intervention. PCI is effective in restoring perfusion in patients with acute STEMI and, if performed by experienced operators in dedicated medical centers, has been shown in multiple trials to provide a greater survival benefit and lower risk for serious bleeding compared to thrombolytics. If patients with an acute STEMI present within 2 to 3 hours of symptom onset and can receive PCI within 90 minutes, then PCI is the recommended reperfusion therapy. PCI can also be used in patients with a contraindication to thrombolytic therapy or who are hypotensive or in cardiogenic shock, for whom thrombolytics offer no survival benefit. PCI is accomplished by cardiac catheterization, in which a guidewire is inserted into the occluded coronary artery and a small balloon threaded over the guidewire and inflated in an attempt to open the blockage and restore blood flow. Sometimes intraluminal expandable stents are deployed, which may improve vessel patency. Use of primary PCI may be limited by the availability of the facilities and personnel required to perform the procedure in a timely fashion.
Thrombolytics. If PCI is not available, patients with STEMI should receive thrombolytics, which have been shown to reduce mortality and preserve
Figure 3–3. Sample algorithm for assessment and treatment of chest pain.
myocardial function. In patients without ST-segment elevation, thrombolytics have not demonstrated the same mortality benefit. Because myocardium can be salvaged only before it is irreversibly injured (“time is muscle”), patients benefit maximally when the drug is given early (eg, within 1-3 hours after the onset of chest pain), and the relative benefits decline with time. The major risk of thrombolytics is bleeding, which can lead to potentially disastrous situations, such as intracranial hemorrhages. The risk of hemorrhage is relatively constant, so the risk begins to outweigh the benefit by 12 hours, at which time most infarctions are completed, and the at-risk myocardium is dead. Thrombolytic therapy is indicated if all of the following criteria are met:
- Clinical complaints are consistent with ischemic-type chest pain.
- ST-segment elevation more than 1 mm is present in at least two anatomically contiguous leads.
- There are no contraindications to thrombolytic therapy.
- Patient is younger than 75 years (greater risk of hemorrhage if > 75).
Contraindications to thrombolytics are related to the patient’s bleeding risk and include recent major surgery, active internal bleeding, suspected aortic dissection, severe hypertension, or a prior history of a hemorrhagic stroke.
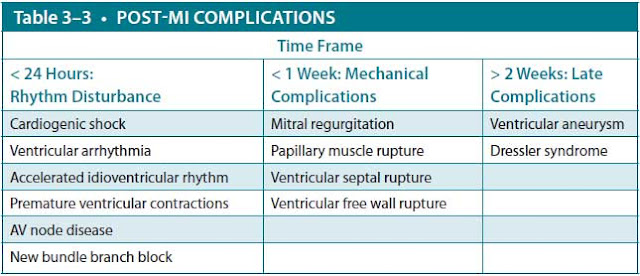
Complications
In acute MI, mortality is usually a result of ventricular arrhythmias or myocardial pump failure and resultant cardiogenic shock (Table 3–3).
Ventricular Arrhythmias. Life-threatening ventricular arrhythmias, such as ventricular tachycardia (VT) and ventricular fibrillation (VF), are common, especially in the first 24 hours. Historically, most deaths from acute MI occurred in the first hour and were caused by VT/VF. This has diminished in recent years with earlier, more aggressive treatment of ischemia and arrhythmias. Premature ventricular contractions (PVCs) are very common but generally not treated with antiarrhythmic agents unless they occur very frequently, are sustained, or induce hemodynamic
compromise. Sustained VT (> 30 seconds) and VF are life threatening because they prevent coordinated ventricular contraction, causing cardiovascular collapse. They are treated with defibrillation, followed by infusion of intravenous antiarrhythmics such as amiodarone. Electrolyte deficiencies, such as hypokalemia or hypomagnesemia, can potentiate ventricular arrhythmias and should be corrected.
One benign ventricular arrhythmia that is generally not suppressed by antiarrhythmics is the accelerated idioventricular rhythm. This is a wide-complex escape rhythm between 60 and 110 bpm that frequently accompanies reperfusion of the myocardium but causes no hemodynamic compromise.
Supraventricular and Atrial Tachyarrhythmias. Supraventricular or atrial tachyarrhythmias are much less common after acute MI, but they can worsen ischemia and cause infarct extension as a consequence of the rate-related increase in myocardial oxygen demand. When they cause hemodynamic instability, they also are treated with immediate direct current (DC) cardioversion.
Bradyarrhythmias. Other frequent rhythm disturbances are bradyarrhythmias. Sinus bradycardia is frequently seen in inferior MI because the RCA supplies the sinoatrial node, but the condition generally requires no treatment unless it causes hypotension. If the heart rate is slow enough to cause cardiac output and blood pressure to fall, intravenous atropine is usually administered.
Bradyarrhythmias can also be caused by atrioventricular (AV) conduction disturbances. First-degree AV block (PR interval prolongation) and Mobitz I second-degree AV block (gradual prolongation of the PR interval before a nonconducted P wave) are often caused by AV nodal dysfunction, for example, nodal ischemia caused by inferior MI. Patients who are symptomatic can be treated with atropine.
Conduction Dysfunctions. Conduction disturbances below the AV node typically produce a widened QRS complex. Examples include Mobitz II second-degree AV block (nonconducted P waves not preceded by PR prolongation) and third-degree AV block (complete AV dissociation with no P-wave conduction). Third-degree AV block also can be caused by AV nodal dysfunction. These arrhythmias are described more fully in other cases. Conduction disturbances caused by involvement of the bundle of His include LBBB or right bundle branch block (RBBB) with left anterior hemiblock. Conduction disturbances below the AV node generally have a worse prognosis than AV nodal dysfunction because they are generally seen with anterior infarction in which a significant amount of myocardium is damaged. When symptomatic bradycardias such as third-degree AV block develop, they may be treated with external pacing but can require placement of a temporary transvenous pacemaker if the patient is in cardiogenic shock from complete heart block. Patients in persistent complete heart block will require placement of a permanent pacemaker.
Cardiac pump failure and cardiogenic shock. Cardiogenic shock in acute MI usually is the most severe form of LV pump failure, manifested by end-organ hypoperfusion. Ischemic reduction in ventricular diastolic compliance may lead to transient pulmonary congestion, associated with elevated left-sided filling pressures. Extensive myocardial necrosis and less contracting heart muscle may cause systolic failure and reduced cardiac output. Patients with hypotension frequently are evaluated by
pulmonary artery (Swan-Ganz) catheterization to assess hemodynamic parameters. Cardiogenic shock is diagnosed when the patient has hypotension with systolic arterial pressure less than 80 mm Hg, markedly reduced cardiac index less than 1.8 L/min/m2, and elevated LV filling pressure (measured indirectly with a pulmonary capillary wedge pressure > 18 mm Hg). Clinically, such patients appear hypotensive, with cold extremities because of peripheral vasoconstriction, pulmonary edema, and elevated jugular venous pressure, reflecting high left- and right-sided filling pressures. Supportive treatment includes hemodynamic monitoring, adequate ventilation and oxygenation, and blood pressure support with vasopressors such as dobutamine and dopamine. These patients also may require mechanical assistance to augment blood pressure while providing afterload reduction, using intra-aortic balloon counterpulsation. Cardiogenic shock may require urgent revascularization with primary PCI or coronary artery bypass surgery.
Hypotension may also be seen in patients with right ventricular (RV) infarction, which is a complication of RCA occlusion and inferior infarction. In this case, LV function is not impaired, but LV filling is dramatically reduced because of the right-sided ventricular failure (the left heart can only pump out what it receives from the right heart). These patients can be recognized clinically as hypotensive, with markedly elevated jugular venous pressure but clear lung fields and no pulmonary edema seen radiographically (in contrast to the pulmonary edema seen in patients with
hypotension to LV failure). The diagnosis is confirmed by observation of ST-segment elevation in a right-sided ECG. In this setting, RV function is impaired and highly dependent on adequate preload, so treatment consists of volume replacement with crystalloid or colloid solution. Diuretics or nitrates should be avoided in these patients because they lower preload, which can cause catastrophic cardiovascular collapse. Patients with RV dysfunction or failure may require inotropic support to increase blood delivery to the LV.
Mechanical Problems. A number of mechanical problems can complicate acute MI, usually presenting within the first week with a new systolic murmur. The most common is papillary muscle dysfunction caused by LV ischemia or infarction, leading to mitral regurgitation, which may or may not be hemodynamically significant. This is in contrast with papillary muscle rupture, which produces a flail mitral leaflet and acute mitral regurgitation with development of heart failure and cardiogenic shock. Ventricular septal rupture can also occur, possibly leading to development of acute heart failure and shock. Transthoracic echocardiography can be used to distinguish among these conditions. In all of them, stabilization of cardiogenic shock is accomplished using afterload reduction with intravenous nitroglycerin or nitroprusside and sometimes with intra-aortic balloon counterpulsation until definitive, urgent, surgical repair can be accomplished. Other modalities of mechanical circulatory support may be used for temporary support in cardiogenic shock, including a LV assist device or venous-arterial extracorporeal membrane oxygenation (ECMO).
The most catastrophic mechanical complication is rupture of the ventricular free wall. As blood fills the pericardium, cardiac tamponade develops rapidly, with sudden pulselessness, hypotension, and loss of consciousness. This complication nearly always is fatal.
Post-MI Risk Stratification. Post-MI, patients should be stratified to identify patients who are at high risk for subsequent cardiac events and who might benefit from revascularization. The initial evaluation involves noninvasive testing. Submaximal exercise stress testing is generally performed in stable patients before hospital discharge to detect residual ischemia or ventricular ectopy and to provide a guideline for exercise in the early recovery period. Evaluation of LV systolic function, usually with echocardiography, is routinely performed. High-risk patients include those with impaired systolic function, large areas of ischemic myocardium on stress testing, postinfarction angina, or ventricular ectopy. These patients might benefit from coronary angiography to evaluate for revascularization. PCI can be performed to reduce anginal symptoms, and coronary artery bypass surgery should be considered for patients with multivessel atherosclerotic stenosis and impaired systolic function because the surgery may reduce symptoms and prolong survival. Post-STEMI patients with LV dysfunction (LV ejection fraction [EF] < 40%) are at increased risk for sudden cardiac death from ventricular arrhythmias and may benefit from placement of an implanted cardioverter-defibrillator (ICD).
Secondary Prevention of Ischemic Heart Disease. Medical therapy to reduce modifiable risk factors is the cornerstone of post-MI care. In addition to symptom relief, the major goal of medical therapy is to prevent cardiac events: fatal or nonfatal MI. By far, the most important risk factor is smoking cessation. Quitting tobacco use can reduce the risk of fatal or nonfatal cardiac events by more than 50%, more than any other medical or surgical therapy available.
A number of other therapies reduce the risk of recurrent cardiovascular events and prolong survival in patients with coronary artery disease. Antiplatelet agents such as aspirin and clopidogrel reduce the risk of thrombus formation. Beta-blockers reduce myocardial oxygen demand and may help suppress ventricular arrhythmias. Statins improve serum cholesterol levels and may stabilize vascular plaque, reducing the number of coronary events and prolonging survival. Angiotensin-converting enzyme (ACE) inhibitors decrease ventricular remodeling and reduce mortality and are recommended for all patients after STEMI. Aldosterone antagonists such as spironolactone or eplerenone reduce mortality in patients with LV EF < 40% and clinical heart failure or diabetes. Finally, screening for depression is appropriate, as depression is common (∼20%) post-MI and has been associated with increased rates of hospitalization and death.
CASE CORRELATION
- See also Case 2 (Metabolic Syndrome), Case 10 (Acute Pericarditis Caused by Systemic Lupus Erythematosus), and Case 13 (Limb Ischemia, Peripheral Vascular Disease)
COMPREHENSION QUESTIONS
3.1 A 36-year-old woman has severe burning chest pain that radiates to her neck. The pain occurs particularly after meals, especially when she lies down, and is not precipitated by exertion. She is admitted for observation. Serial ECG and troponin I levels are normal. Which of the following is the best next step?
A. Stress thallium treadmill test
B. Initiation of a proton pump inhibitor
C. Coronary angiography
D. Initiation of an antidepressant such as a selective serotonin reuptake inhibitor (SSRI)
3.2 A 56-year-old man is admitted to the hospital for chest pain of 2-hour duration. His heart rate is 42 bpm, with sinus bradycardia on ECG, as well as ST-segment elevation in leads II, III, and aVF. Which of the following is the most likely diagnosis?
A. Left-circumflex territory infarction
B. Inferior wall infarction
C. LV aneurysm
D. Anterior wall infarction
3.3 A 59-year-old diabetic woman had suffered an acute anterior wall MI. Five days later, she gets into an argument with her husband and complains of chest pain. Her initial ECG shows no ischemic changes, but serum cardiac troponin I levels are drawn and return mildly elevated at this time. Which of the following is the best next step?
A. Use thrombolytic therapy.
B. Treat with percutaneous coronary intervention.
C. Perform coronary artery bypass.
D. Perform serial ECGs and obtain CK-MB.
3.4 A 59-year-old male smoker complains of severe retrosternal squeezing chest pain of 30 minutes’ duration. The paramedics have given sublingual nitroglycerin and oxygen by nasal cannula. His blood pressure is 110/70 mm Hg, and heart rate is 90 bpm on arrival to the Emergency Department. The ECG is normal. Which of the following is the best next step?
A. Echocardiography
B. Thallium stress test
C. Aspirin
D. Coronary angiography
E. Coronary artery bypass
ANSWERS
3.1 B. It is appropriate to evaluate chest pain to first rule out cardiac ischemia. One of the most common causes of “chest pain,” particularly in a younger patient, is gastroesophageal reflux or esophageal spasm. This patient has classic symptoms of reflux esophagitis and is best treated with a proton pump inhibitor. If the chest pain has the characteristics of angina pectoris (retrosternal location, precipitated by exertion, relieved by rest or nitroglycerin), it should be investigated with a stress test (answer A) or coronary angiography (answer C). SSRIs may be used to treat panic disorder, which can present with chest pain and palpitations; however, there are no indications for an SSRI in this patient (answer D).
3.2 B. Sinus bradycardia is often seen with inferior wall MI because the RCA supplies the inferior wall of the LV and the sinoatrial node. The ischemic changes in leads II, III, and aVF are in the region of the inferior leads. A circumflex-territory infarction (answer A) usually leads to a lateral wall infarction, involving leads I, aVL, V5, and V6. LV aneurysm (answer C) can be a late complication of MI, associated with persistence of ST elevation for weeks after an MI. Anterior wall infarction (answer D) is associated with ST elevation in leads V1 through V4.
3.3 D. Diabetic patients can have myocardial ischemia or infarction with atypical or absent symptoms. Clinical suspicion and a liberal use of cardiac enzyme testing are required. Troponin levels often remain elevated for 7 to 10 days and should not be used to diagnose reinfarction, especially if the levels are trending downward. New ECG findings or rapidly rising markers such as serum myoglobin or CK-MB can be used in this setting. Thrombolytics, PCI, or coronary artery bypass grafting (CABG) (answers A, B, and C) are not indicated in this patient at this time, as her elevated troponins are likely due to prior infarct, and she does not meet criteria for STEMI or NSTEMI currently.
3.4 C. Aspirin is the first agent that should be used after oxygen and nitroglycerin. Aspirin use decreases mortality in the face of an acute coronary event. Because initial ECGs and cardiac enzymes may be normal in an acute MI, serial studies are needed to definitively rule out MI. Echocardiography or coronary angiography (answers A and D) may be appropriate in working up this patient’s chest pain, but the first priority is to administer aspirin. Stress tests (answer B) are used to determine if patients with moderate pretest probability of coronary artery disease have it or not. Stress tests are never part of the acute management of cardiac chest pain. CABG (answer E) is premature in this patient, who does not have any indications for the procedure at this time.
CLINICAL PEARLS
▶ Acute coronary syndromes (unstable angina or acute MI) occur when a thrombus forms at the site of rupture of an atherosclerotic plaque and acutely occludes a coronary artery.
▶ Acute MI is diagnosed based on the presence of at least two of three criteria: typical symptoms, ECG findings, and cardiac enzymes.
▶ ECG findings can further stratify MI into STEMI or NSTEMI. Initial ECG and enzyme levels may be normal, so serial studies are necessary.
▶ Typical initial treatment of ACS includes aspirin, beta-blockers, nitrates, heparin, and morphine. Nitrates and beta-blockers should be used cautiously as they lower cardiac output.
▶ Early reperfusion with PCI or thrombolytics reduces mortality and preserves ventricular function in patients who have ST-segment elevation, have no contraindications, and receive treatment within the first 6 to 12 hours.
▶ The goal of secondary prevention after MI is to prevent recurrent cardiac events and death. Smoking cessation, antiplatelet medications, beta-blockers, ACE inhibitors, statins, and aldosterone antagonists all reduce the rate of events and mortality.
▶ After MI, PCI can be performed to reduce ischemia and anginal symptoms. Bypass surgery may be indicated for patients with multivessel stenosis and impaired systolic function to reduce symptoms and prolong survival.
▶ The ECG can indicate the location of the ischemia or infarction: anterior (leads V2 through V4); lateral (leads I, aVL, V5, and V6); inferior (leads II, III, and aVF); and posterior (R waves in leads V1 and V2).
▶ STEMI is characterized by ischemic discomfort along with ST-segment elevation on ECG and biomarkers.
▶ Unstable angina and NSTEMI will not have ST-segment elevation; NSTEMI is diagnosed by positive cardiac biomarkers.
REFERENCES
American College of Physicians and the Clerkship Directors in Internal Medicine. Acute coronary syndrome. In: Alguire P, ed. Internal Medicine Essentials for Students. Chicago, IL: Donnelley; 2011:10-13.
Antman EM, Loscalzo J. ST-segment elevation myocardial infarction. In: Jameson JL, Fauci AS, Kasper D, et al, eds. Harrison’s Principles of Internal Medicine. 20th ed. New York, NY: McGraw Hill; 2018:2021-2035.
Antman EM, Selwyn AP, Loscalzo, J. Ischemic heart disease. In: Jameson JL, Fauci AS, Kasper D, et al, eds. Harrison’s Principles of Internal Medicine. 20th ed. New York, NY: McGraw Hill; 2018:1998-2015.
Kushner FG, Hand M, Smith SC Jr, et al. 2009 focused update of the ACC/AHA 2004 guidelines for the management of patients with ST-elevation myocardial infarction. Circulation. 2009;120:2271.
Tatum JL, Jesse RL, Kontos MC, et al. Comprehensive strategy for the evaluation and triage of the chest pain patient. Ann Emerg Med. 1997;29:116-125.





0 comments:
Post a Comment
Note: Only a member of this blog may post a comment.