Olfaction Case File
EUGENE C.TOY, MD, RAHUL JANDIAL, MD, PhD, EVAN YALE SNYDER, MD, PhD, MARTIN T. PAUKERT, MD
CASE 21
A 22-year-old male is involved in a motorcycle accident and suffers a closed head injury with bilateral paramedian frontal lobe contusions. After a lengthy stay in the intensive care unit, he enters a rehabilitation facility and begins to make significant progress. He begins to notice that he is unable to taste any of his food and unable to smell his coffee. On examination he is noted to have complete absence of the ability to smell. An MRI is obtained 3 months after his injury which demonstrates encephalomalacia in the paramedian frontal lobe regions corresponding to the area of his prior contusions. The patient is diagnosed with posttraumatic olfactory dysfunction.
- What is the mechanism of this patient’s dysfunction?
- What bony anatomic structure is likely damaged?
ANSWERS TO CASE 21: OLFACTION
Summary: A 22-year-old male with a history of a closed head injury following a motorcycle accident. After a period of neurological recovery, the patient notes an inability to smell. Neuroimaging reveals posttraumatic changes in the paramedian frontal lobes, which is in the region of the olfactory bulbs and tracts.
- Mechanism of posttraumatic olfactory dysfunction: Posttraumatic olfactory dysfunction may be caused by a shearing injury of the olfactory nerve filaments, or by brain contusion and hemorrhage within the olfactory brain regions.
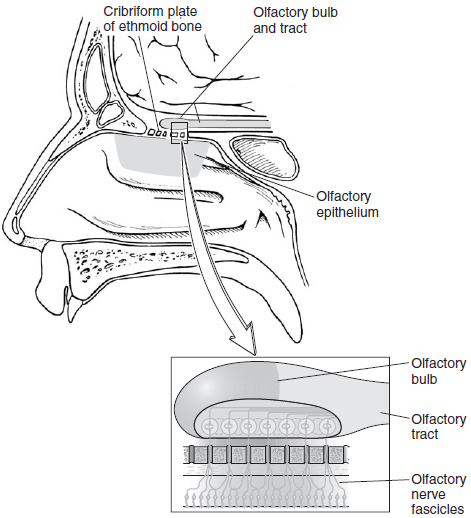
- Bony anatomic site: The cribriform plate of the naso-orbito-ethmoid region is commonly affected in this case.
CLINICAL CORRELATION
Posttraumatic anosmia is a common finding in patients that have suffered head injuries. The axons of olfactory receptor cells are delicate and pass through small foramina of the cribriform plate at the base of the skull and synapse directly in the olfactory bulb. Any tearing or shearing of the axons during the trauma can result in olfactory dysfunction. It may occur with fractures of the naso-orbito-ethmoid region, involving the cribriform plate, or with rapid translational shifts in the brain secondary to coup or contracoup forces generated by blunt head trauma. Head trauma often results in traumatic brain injury in the form of cortical contusion or intraparenchymal hemorrhage. Contusion of the olfactory bulbs or cortical lesions at the olfactory brain regions (amygdala, temporal lobe region, frontal lobe region) can lead to posttraumatic anosmia. The treatment is expectant observation.
APPROACH TO OLFACTION
Objectives
- Know the anatomic structures involved in the olfactory system.
- Be familiar with the cortical areas involved in olfactory associations.
- Be able to name and describe some of the etiologies of olfactory dysfunction.
Definitions
Mitral cells: Second-order neurons of the olfactory system. They receive information from olfactory receptor neurons through synapses in the olfactory bulb, their axons project as the olfactory tract.Entorhinal cortex: An important memory center of the brain located in the medial temporal lobe. Mitral cells project to this region of the cortex and are thought to contribute to emotional associations with olfaction.Anosmia: Absence of the sense of smell.Hyperosmia: Abnormally acute smell function. Olfactory agnosia: Inability to recognize an odor sensation.Dysosmia: Distorted smell perception.
DISCUSSION
Olfaction allows us to detect and discriminate between a great variety of odors within our environment. The olfactory system consists of several elements of the central nervous system: the olfactory nerves, bulbs, and tracts, the olfactory tubercle, the primary olfactory cortex, the entorhinal cortex of the parahippocampal gyrus, and the amygdala. The functions of this system include distinguishing isolated odors from other background odors, determining the concentration of the odor and being able to identify it at different concentrations, creating a representation of the odor, and pairing the odor with an associated memory. For many other species, olfaction is important for locating food and communication.
Olfactory stimuli are sensed by specialized peripheral olfactory receptors in a part of the nasal mucosa called the olfactory epithelium. This specialized tissue consists of pseudostratified columnar olfactory epithelium located on the superior concha, the roof of the nasal chamber, and the upper portion of the nasal septum. The receptor cells are bipolar neurons with cilia at their dendritic endings in the olfactory epithelium. Interestingly, olfactory neurons are the only special sensory receptors that are the nerve itself. The molecules that are smelled or tasted alter the membrane potential of the receptors through ligand-receptor-mediated second messenger mechanisms that result in ion channel openings in the ciliary membrane. There are more than 1000 different receptor proteins in the olfactory cilia, with each neuron expressing a single receptor. Olfactory neurons are also unique in that they are short-lived neurons with an average life span of 30-60 days (see Figure 21-1). Aside from perceiving and distinguishing odors, they contribute to the sensation of taste.
The ability to discriminate between different odorants is the result of differential expression of the receptor proteins in receptor cells across the surface of the mucosa, combined with selective convergence of axons from functionally related receptor cells to target cells in the olfactory bulb. These axons are grouped into fascicles before passing through the cribriform plate as the olfactory nerve. The olfactory axons terminate in the olfactory bulb in what are called the glomeruli. The olfactory bulbs, which rest on the cribriform plates, contain several types of neurons, including inhibitory interneurons and mitral cells. The mitral cells receive direct synaptic input from olfactory nerve fibers and project their axons in the lateral olfactory tract. Each mitral cell receives input from olfactory nerves expressing the same odorant receptor. Inhibitory interneurons also form synapses with mitral cell dendrites and inhibit mitral cells from surrounding glomeruli, producing lateral inhibition.
Figure 21-1. Basic neural circuits in the olfactory bulb. (With permission from Martin JH. Neuroanatomy: Text and Atlas. 3rd ed. New York, NY: McGraw-Hill; 2003: 217.)
The anterior olfactory nucleus is located in the olfactory stalk and contains groups of neurons. The olfactory portion of the anterior commissure originates in the olfactory stalk as one of these groups. Information from the ipsilateral olfactory bulb is received and transmitted to the contralateral olfactory bulb by way of the anterior commissure through this group of neurons. The olfactory stalk is positioned in the olfactory sulcus of the frontal lobe, directly lateral to the gyrus rectus of the orbitofrontal cortex. The olfactory tract bifurcates here into medial and lateral striae. A portion of the fibers in the medial olfactory stria contain the axons of the anterior olfactory nucleus neurons which cross the anterior commissure to the contralateral olfactory bulb. The remaining fibers consist of the mitral cell axons and terminate in the ipsilateral olfactory tubercle within the anterior perforated substance. Mitral cell axons only project ipsilaterally.
The lateral stria, or lateral olfactory tract, consists primarily of mitral cell axons and projects to the lateral margin of the anterior perforated substance, the piriform cortex, a small rostral portion of the entorhinal cortex, and the corticomedial amygdala. This pattern distinguishes the olfactory system as being the only sensory system in which second-order neurons (the mitral cells) project directly to cerebral cortex, and not the thalamus.
The primary olfactory cortex, or piriform cortex, is the key region involved in the conscious perception of smell. Lesions in this region have been shown to result in failure to discriminate between various odorants. The piriform cortex is also unique in that it consists of a three-layered cortex, making it phylogenetically older than the six-layered cortex of the visual, auditory, and somatosensory systems. The olfactory cortex has reciprocal thalamocortical connections used for discriminative functions. Projections travel directly from the primary olfactory cortex to the lateral orbitofrontal cortex and indirectly through the magnocellular portion of the dorsomedial nucleus of the thalamus. Other communications important in olfactory discrimination include cortococortical connections between the temporal pose and the orbitofrontal cortex.
Olfactory impulses that reach the corticomedial amygdala are important in many species for the control of social behaviors. In humans, however, the behavioral significance of olfactory projections to the amygdalae has not been clarified. The function of the olfactory input to the hippocampus through the entorhinal cortex is also unclear. These pathways, however, are thought to integrate olfactory information with visual, auditory, and somatosensory impulses arriving from other association cortices. Through connections with the amygdala, the hippocampus is thought to participate in the integration of multisensory inputs into appropriate emotional and physiological responses to external stimuli. This may contribute to developing emotional responses to specific odors.
Anosmia, the loss of sense of smell, can result from several etiologies. Traumatic injuries that injure the frontal lobes can affect the primary olfactory cortex. Fractures of the cribriform plate can result in injury to the olfactory bulbs or tracts. Infections such as the common cold, viral hepatitis, syphilis, bacterial meningitis, cerebral abscesses, or osteomyelitis of the frontal or ethmoid regions can also injure the olfactory system. The most frequent cause of smell loss in adults is an upper respiratory tract infection (URI). The URI is often severe, and the smell loss is most commonly partial. Direct insult to the olfactory neuroepithelium is the primary basis of the problem. Viruses can cause edema and hyperemia of nasal membranes, necrosis of cilia, and cellular destruction. And they can produce varying degrees of destruction. Biopsy studies of olfactory epithelia from patients with post-URI anosmia showed greatly reduced numbers of olfactory receptors. The injury can be followed by resolution of infection and regeneration. Anosmia results when there is lack of regeneration secondary to severe destruction of the epithelium. If there is patchy destruction, patients may have hyposmia. If the regeneration of receptor neurons and their central attachments are “misguided” to reach abnormal locations in the brain, patients may experience dysosmia, or distorted smell. In addition to direct insults, many viruses can invade the
CNS via the olfactory neuroepithelium to cause subsequent dysfunction. No effective treatment has been found for post-URI hyposmia. Even though spontaneous recovery in some patients is theoretically possible, meaningful recovery is rare when marked loss has been present for a period of time. Less common causes of anosmia include olfactory groove meningiomas, frontal lobe gliomas, metabolic diseases, amphetamine or cocaine use, and Parkinson and Alzheimer disease. A complete anosmia will result in the ability to recognize flavors as the olfactory and taste systems function together in the perception of flavors. Hyperosmia, an increase in olfactory sensitivity, frequently occurs in early pregnancy and can also occur in conversion disorders and some psychoses.
COMPREHENSION QUESTIONS
Refer to the following case scenario for questions 21.1-21.2:
A 37-year-old man comes into your office with the complaint that he cannot smell. He states that several months ago he was involved in a bar fight in which his nose and several other facial bones were fractured. Ever since he was discharged from the hospital at that time, he has not been able to smell.
[21.1] Olfactory neurons are vulnerable to damage as they pass through which opening in the skull?
A. Inferior orbital fissure
B. Foramen ovale
C. Cribriform plate
D. Internal nasal valve
[21.2] Which of the following statements best describes this man’s olfactory neurons prior to his injury?
A. The maximal numbers of olfactory neurons are present at birth, and the number slowly decreases throughout life as neurons are damaged.
B. Each olfactory neuron expresses receptors for only one odorant.
C. Olfactory neurons project directly to the olfactory cortex.
D. Olfactory neurons expressing different but complimentary odorants synapse on the same mitral cell.
[21.3] A 33-year-old woman comes into your office with the complaint that she smells things that others do not smell. This occurs at unpredictable times, typically lasts for several minutes, and then goes away. The smell is typically something burning. You suspect that this woman is having simple partial seizures, and order an EEG. An abnormal discharge in which brain region would confirm your findings?
A. Occipital lobe
B. Superior parietal lobe
C. Medial temporal lobe
D. Anterolateral frontal lobe
Answers
[21.1] C. As the olfactory neurons pass through the tiny holes in the cribriform plate, they are susceptible to injury, as occurred in this case. Olfactory neurons are small, bipolar neurons that run only a very short way from the olfactory epithelium of the superior nasal cavity through the cribriform plate to the olfactory bulb in the olfactory groove of the frontal lobe. Depending on the degree of damage to the olfactory epithelium and the cribriform plate, the neurons may be able to regenerate, resulting in a return of smell.
[21.2] B. Although there are thousands of odorant receptors expressed in humans, each olfactory neuron expresses only one odorant receptor. The neurons expressing the same receptor project through the cribriform plate and synapse on mitral cells in the olfactory bulb. Each mitral cell receives input from neurons expressing the same odorant. Mitral cells then project their axons down the olfactory tract directly to the olfactory cortex. The neurons in the olfactory epithelium have a lifespan of only several months, after which time they die and are replaced by new olfactory neurons derived form the basal cell layer of the olfactory epithelium.
[21.3] C. The olfactory cortex, made up of the piriform cortex and the periamygdaloid cortex is located on the medial aspect of the temporal lobe. Abnormal EEG readings from this area during the experience of an abnormal smell would confirm that seizure discharge is responsible for this woman’s symptoms. The occipital lobe houses the primary visual cortex; discharge here would result in abnormal vision. The superior parietal lobe contains the primary sensory cortex, and a discharge here would result in abnormal sensation. The anterolateral frontal lobe is not a sensory area, but is involved in executive function.
|
NEUROSCIENCE
PEARLS
❖ Olfactory receptor neurons detect odorants in the olfactory
epithelium in the nose and transmit information
through the cribriform plate to mitral cells in the olfactory
bulbs.
❖ The olfactory system is the only sensory system in which
secondorder neurons (the mitral cells) project directly
to cerebral cortex.
❖ The projection of olfactory information to the limbic area
explains why certain smells can be evocative of
memory and emotion.
❖ Anosmia can result from traumatic, infectious, and neoplastic etiologies. |
REFERENCES
Buck LB. Smell and taste: the chemical senses. In: Kandel ER, et al, Principles of Neural Science. 4th ed. New York, NY: McGraw-Hill; 2000.
Martin JH. The olfactory system. Neuroanatomy, Text and Atlas. 2nd ed. Stamford, CT: Appleton & Lange; 1996.
Ropper AH, et al. Disorders of smell and taste. Adam’s and Victor’s Principles of Neurology. 8th ed. New York, NY: McGraw-Hill; 2005.

0 comments:
Post a Comment
Note: Only a member of this blog may post a comment.