Axonal Injury Case File
EUGENE C.TOY, MD, RAHUL JANDIAL, MD, PhD, EVAN YALE SNYDER, MD, PhD, MARTIN T. PAUKERT, MD
CASE 37
A 26-year-old right-handed Asian female presents to the clinic with frequent burning and numbness in the palm of her right hand. The symptoms first began at night when the patient would feel the need to “shake out” her right hand, but now her hand frequently feels tingly and weak during the day. She works as an assembler in a manufacturing factory, and the symptoms have begun to interfere with her job. Upon neurological examination, the Tinel test of applying pressure to the palmar aspect of her wrist tested positive (the patient noted a shock-like sensation to her fingers). There was no indication of neurological
deficit in any other area of the body. The patient also has a history of diabetes, and was ultimately diagnosed with carpel tunnel syndrome.
- What nerve is likely affected?
- What is the pathophysiological mechanism of her symptoms?
ANSWERS TO CASE 37: AXONAL INJURY
Summary: A 26-year-old Asian female complains of progressive pain in the palm of her right hand.
- Nerve affected: Median nerve.
- Disease mechanism: Carpal tunnel is the result of increased pressure and compression of the median nerve and tendons in the carpal tunnel. The syndrome is usually owing to having a smaller carpal tunnel, trauma and injury to the wrist, work stress, or other mechanical problems of the wrist joint.
CLINICAL CORRELATION
Carpal tunnel is more prevalent among populations of women, diabetics, and assembly-line workers because of a smaller carpal tunnel, related nerve effects that increase susceptibility to compression, and increased wrist trauma, respectively. This is a peripheral nerve disorder caused by a less obvious manifestation of axonal injury. The compression of the median nerve may have been traumatic enough to induce intrinsic mechanisms of axon cell death discussed below and in some cases can be severe enough to sever the axon, but leave the basal lamina sheath of nerve intact to guide regeneration. Carpal tunnel represents the most common of the entrapment neuropathologies that are a group of disorders resulting from the chronic compression of peripheral nerves, namely the median nerve, resulting in pain or loss of function. Specifically, entrapment syndromes result from chronic injury to a nerve as it travels through an osseoligamentous tunnel; the compression usually is between ligamentous and bony surfaces. Pathophysiology includes microvascular (ischemic) changes, edema, dislocation of the nodes of Ranvier, and structural alterations in membranes at the organelle levels in both the myelin sheath (ie, focal segmental demyelination), and the axon. Severe cases of entrapment can result in Wallerian degeneration of the axons and permanent fibrotic changes in the neuromuscular junction that prevents reinnervation. If symptoms persist for 6 months or longer following nonsurgical treatment, this may be an indication of the continued physical compression of the nerve by the band of tissue surrounding the wrist that inhibits axon regeneration. The band is cut during surgery to reduce pressure on the median nerve.
APPROACH TO AXONAL INJURY
Objectives
- Be able to understand disorders resulting from the chronic compression of peripheral nerves.
- Be able to alleviate neuronal death and atrophy by experimental application of various trophic factors such as NGF.
Definitions
L-type calcium channel blockers: These blockers inhibit L-type voltagedependent calcium channels. L-type channels have large sustained conductance, inactivate slowly, are responsible for the plateau phase of the action potential, and may trigger release of internal calcium ions.Calpains: These are the calcium ion–dependent proteolytic enzymes that modulate cellular function. Cytoskeleton molecules are the main substrate for calpains, thus, calpain activation causes the degradation of the axonal cytoskeleton. Intuitively, inhibition of calpains can also prevent axonal degeneration in vitro.Target-derived trophic factors: Growth factors derived from the target of intended neuronal growth, for instance, a target neuron from which another will synapse can release target-derived trophic factors to encourage neural growth.Retrograde cell death: Following axonal injury retrograde cell death is the death of the neuron associated with the injured axon, usually following the retraction of the axon away from the severance site.Anterograde cell death: Following axonal injury, anterograde cell death is the death of the neuron that synapses with the injured axon.Nerve growth factor (NGF): It is a small protein secreted from target cells that causes differentiation, survival, and maintenance of sympathetic and sensory neurons.Chromatolysis: The disintegration within a nerve cell body of chromophil substance, such as chromatin. Typically occurs after peripheral cell damage or cell exhaustion.
DISCUSSION
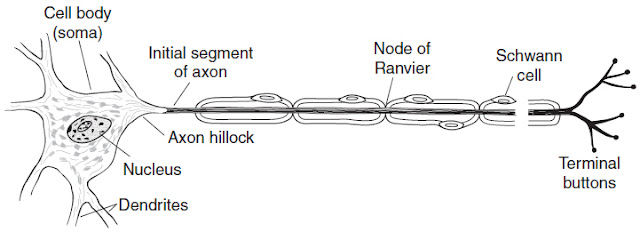
Most nervous system damage will result in some sort of axonal injury. They are uniquely vulnerable, given their elongated physiology and how far removed they are from the cell body, from which they derive proteins for homeostasis and function (see Figure 37-1). When an axon is cut, it is necessarily removed from its source of protein synthesis compromising both the axon and its surrounding myelin. After injury, there is an active inherent mechanism that causes axon death that is not dependent on intervention from outside cells for degeneration. This mechanism of axonal death harkens back to basic neuronal function of synaptic transmission.
Figure 37-1. Motor neuron with myelinated axon. (With permission from Ganong’s Review of Medical Physiology. 22nd ed. Figure 2-2, page 48.)
There are two phases of calcium entry into the axon after injury. A diffusion of calcium from the extracellular pool occurs immediately, and is interrupted once the axonal membrane reseals. The second movement of calcium into the cell precedes axonal degeneration and proceeds through the voltagegated ion channels (L-type channels), as well as the sodium–calcium exchange pump. Entry of calcium through the first route is a consequence of axon depolarization that is a result of the axon losing its ability to maintain a normal resting membrane potential. Additionally, because of this first route of second phase calcium entry, degeneration in sensory axons can be blocked by L-type calcium channel blockers in vitro.
The sodium–calcium exchange pump that drives the second route of calcium entry is actually running in reverse following axonal injury. Normally, allowing the movement of sodium ions down concentration and potential gradients across the membrane drives ATP production that powers the sodium–calcium exchange pump to remove calcium from the cell. However, if the membrane becomes depolarized because of action potential activity or exchange pump failure, and the sodium gradient diminishes then the high concentration of extracellular calcium can drive the sodium–calcium countertransport pump in reverse. The end result is a massive increase in calcium concentration within the axon exceeding the 200-μm threshold for calciumactivated enzymes, calpains, and possibly other mechanisms as well. Cytoskeletal molecules are the main enzymatic substrates of calpains, thus, calpain activation causes the degradation of the axonal cytoskeleton. Intuitively, inhibition of calpains can also prevent axonal degeneration in vitro.
The fallout from axonal injury can also extend beyond the axon distal to the cut to the neuron itself. Neuronal death can occur by apoptosis or necrosis. Death via apoptosis is usually caused by the loss of target-derived trophic factors that provide support to the neuron, or because of the intense influx of calcium mentioned earlier. Rapid necrotic cell death mainly occurs when the axon is damaged very close to the cell body, usually explained by a combination of physical trauma to the membrane and cytoskeleton of the neuron, and, again, the calcium influx that results from axonal membrane disruption. Both neurons in the vicinity of and some distance away from the lesion can be lost via apoptosis days after the injury. Adding on to the retrograde cell death of neurons whose axons have been cut, there is also some anterograde cell death of the neurons to which those damaged axons connect by apoptosis. Neuronal death in both these fashions is at least partly because of loss of access to trophic factors.
Trophic support for a neuron can be derived from several sources including target neurons from which it synapses; neurons that synapse from it; Schwann cells, astrocytes, and oligodendrocytes that contact it at different points; and microglia that cluster around damaged neurons. Following axonal injury, a neuron clearly loses contact with its target cells and also loses their accompanying trophic support. Synapses on the cell soma have also been shown to retract, replaced first by microglia, the astrocytes. A new source of trophic support from the glial cells surrounding the area of damage replaces the support lost from the damaged neuron’s efferent and afferent connections. Astrocytes become reactive in damaged regions of the CNS. Microglia also migrate to these regions where they undergo mitosis and become activated as well, producing a variety of neurotrophic and toxic cytokines. In the PNS, Schwann cells are activated following axonal injury which leads to a rapid accumulation of macrophages that secrete toxic molecules. Furthermore, neuronal death and atrophy can be alleviated by the experimental application of various trophic factors such as NGF.Thus, the overriding consensus is that loss of trophic support results from axonal damage since many neurons following axonal injury die or become atrophic.
Neurons that survive axonal injury undergo a predictable and welldescribed set of changes. Many of these changes are associated with the reinitiation of protein synthesis for axon growth and regeneration. Anatomically, these major changes in the pattern and quantity of protein synthesis are manifested in the neuron as chromatolysis, the dispersal of the large granular condensations of the rough endoplasmic reticulum accompanied by changes in the appearance of the nucleus. Many of the genes that underlie these changes are upregulated or downregulated after axonal injury and have been identified in the following categories: transcription factors, growth-associated proteins, cytoskeletal proteins, growth factor receptors and growth facts, and cytokines.
COMPREHENSION QUESTIONS
Refer to the following case scenario to answer questions 37.1-37.3:
A 17-year-old male is dropped off outside the emergency department by some “friends” after having been shot in the arm. He will not discuss any specifics of the events surrounding the injury, and does not know the caliber or type of bullet he was shot with. On examination there is a through and through wound of the proximal forearm, just distal to the cubital fossa. There is some bleeding from the wound, but surprisingly little, considering the proximity of the wound to numerous relatively large-sized arteries. On examination, however, the patient has no ability to flex any of his fingers, and weak flexion of his wrist. He also has sensory loss of his volar forearm and his palmar thumb, index, and middle finger. The physician suspects that he has a traumatic transaction of his median nerve.
[37.1] The entry of what ion into the axon distal to the transaction will activate cellular digestive enzymes that will degrade the cytoskeleton?
A. SodiumB. ChlorideC. MagnesiumD. Calcium
[37.2] In addition to degeneration of the axon distal to the site of injury, the entire nerve proximal to the injury may die as well. The absence of what substance, normally transported to the cell body via retrograde transport down the axon can account for this cellular demise?
A. Neurotransmitter vesicle proteinsB. Unused peptide neurotransmittersC. ATPD. Neurotrophic factors
[37.3] Following nerve injury in the peripheral nervous system, what glial cell type surrounding damaged neurons becomes active?
A. Schwann cellsB. AstrocytesC. MicrogliaD. Macrophages
Answers
[37.1] D. Calcium enters into the axonal cytoplasm following injury by two mechanisms. Initially, it enters through the disruption in the cellular membrane by simple diffusion, but this method is stopped
as the membrane reseals. The second method is through voltagegated calcium channels that open as the neuron depolarizes since it is cut off from the cell body, and via the sodium–calcium exchange pump that runs in reverse in this pathologic condition. The calcium in the cell quickly reaches threshold for activation of calpains, which begin to degrade cellular proteins, cytoskeletal elements in particular.
[37.2] D. This cellular demise is because of absence of neurotrophic factors necessary for neuron survival. Normally, a neuron can derive trophic support for a variety of places, including the cell it innervates. Trophic support is the supplying of the neuron with small molecules, like NGF, that are necessary for the survival of the cell. When a neuron is cut off form its supply of trophic factors, as is the case with axonal injury, apoptotic pathways within the cell become activated, resulting in cell death.
[37.3] A. Schwann cells, the primary supporting glial cell of the PNS, become activated following axonal damage. When activated they secrete a variety of cytokines, some of which can serve as neurotrophic factors which can prevent apoptotic neuronal death. They also secrete cytokines that are chemotactic for macrophages, which help to clear the damaged axon, but are not themselves glial cells. Astrocytes and microglia are both active around damaged neurons in the CNS.
|
NEUROSCIENCE
PEARLS
❖ Death of neurons via apoptosis is usually because of the loss of target-derived trophic factors or to the
intense influx of calcium especially after axonal membrane
disruption.
❖ Both neurons in the vicinity of and some distance away from the lesion can be lost via apoptosis days after
the injury.
❖ Neuronal death and atrophy can be alleviated by the experimental application of various trophic
factors such as NGF. |
REFERENCES
Kandel E, Schwartz J, Jessell T, eds. Principles of Neural Science. 5th ed. New York, NY: McGraw-Hill; 2000.
Purves D, Augustine GJ, Fitzpatrick D, et al, eds. Neuroscience. 3rd ed. Sunderland, MA: Sinauer Associates, Inc.; 2004.
Zigmond MJ, Squire LR, Bloom FE, Landis SC, Roberts JL, eds. Fundamental Neuroscience. 2nd ed. San Diego, CA: Academic Press; 1999.

0 comments:
Post a Comment
Note: Only a member of this blog may post a comment.